The quantity PVkBT represents the (kB:Boltzmann constant)
number of molecules of the gas.
mass of the gas.
number of moles of the gas.
translational energy of the gas.
The correct Answer is:A
PV=nRT
n=PVRT
no. of molecules =NA×PVRT
=(PVkBT),(kB=RNA)=Boltzmann constant.
Topper's Solved these Questions
Similar Questions
The major product 'x' formed in the following reaction is:
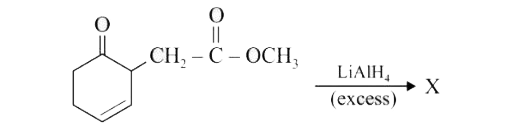
View SolutionFor following reactions
A400K−−→ Product
A200K−−−−−→catalyst Product
it was found that the Ea is decreased by 20 kJ/ mol in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is ( Assume per exponential factor is same ) :
View SolutionThe quantity (PV/KBT) represents
View SolutionThe dimensions of Stefan-Boltzman constant sigma can be written in terms of Plank's constant h. Boltzmann constant k_(B) and the speed of light c as sigma = h^(alpha) K_(B)^(b) c^(Y) . Here
View SolutionThe dimensions of Stefan-Boltzmann constant σ can be written in terms of Planck's constant h, Boltzmann constant kB and the speed of light c as σ=hαkβBcγ. Here
View SolutionThe quantity (PV)/(k_(B)T) represents the ( k_(B): Boltzmann constant)
View SolutionIn the equation (1pβ)=ykBT, where p is the pressure, y is the distance, kB is Boltzmann constant and T is the tempreture. Dimensions of β are
View SolutionThe Boltzmann constant (K_(B)) is:
View SolutionIf A represents Boltzmann constant B represents Planck's constant and C represents speed of light in vacuum then the quantity having the dimensions of A^(4)B^(-3)C^(-2) is
View SolutionThe hydrolysis constant of ammonium acetate is given by A) (K_(w))/(K_(a) ) B) (K_(w))/(K_(b) ), C) (K_(w))/(K_(a)*K_(b) )
View SolutionIn the equation ((1)/(Pbeta))=(y)/(K_(B)T) when P is the pressure y is the distance k_(B) is Boltzmann constant and T is the temperature . Dimensions of are
View SolutionThe quantity (PV)/(k_(B)T) represents the ( k_(B): Boltzmann constant)
View SolutionIf KB is the Boltzmann constant then the quantity (PV/KBT) represents the:
View SolutionBoltzmann Constant, K = R/N where N is Avogadro. number. Which quantity does PV/kT represent?
View SolutionBoltzmann Constant, K = R/N where N is Avogadro. number. Which quantity does PV/kT represent?
View SolutionThe quantity hv/kB corresponds to
View Solution

