The decreasing order of acidic strength of the following is
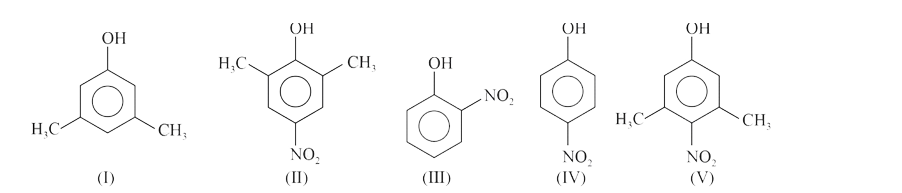
IV>III>I>II>V
IV>III>II>V>I
II>V>IV>III>I
III>V>II>I>IV
The correct Answer is:B
−NO2 shows −I,−M at ortho, para and only −I at meta position. −CH3 groups shows +I hyperconjugation at ortho, para and shows only +I at meta.
Topper's Solved these Questions
Similar Questions
The major product 'x' formed in the following reaction is:
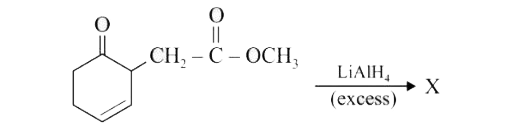
View SolutionFor following reactions
A400K−−→ Product
A200K−−−−−→catalyst Product
it was found that the Ea is decreased by 20 kJ/ mol in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is ( Assume per exponential factor is same ) :
View SolutionArrange the following compounds in decreasing order of their acidic strength.
View SolutionArrange the following in decreasing order of acidic strength
View SolutionArrange the following in order of their decreasing acidic strength ?
View SolutionArrange the following in decreasing order of acidic strength:
View Solutionनिम्न अक्सों-अम्लों में अम्लीय शक्ति का सही घटता हुआ क्रम हैं:
View SolutionThe decreasing order of acidic strength of the following is

View Solutionनिम्न ऑक्सोअम्लों के लिये अम्ल शक्ति का यथार्थ घटता क्रम होगा
View SolutionArrange the following in the decreasing order of acid strength ?
View SolutionArrange the following in the decreasing order of acid strength ?
View SolutionThe following compounds will have a decreasing order of acid strength-
View SolutionThe decreasing order of acidic strength will be-
View SolutionThe decreasing order of acid strength of the following acids is-
View SolutionThe decreasing order of acidic strength of the following is:
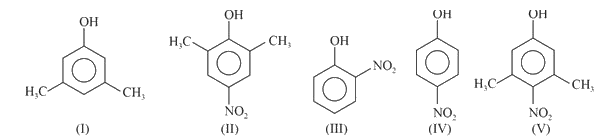
View SolutionArrange the following in the decreasing order of acidic strength
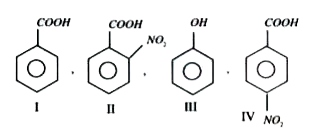
View Solution

