The number of Pi bonds in one molecule of “Aspartame” is ________
The correct Answer is:6
To determine the number of pi bonds in one molecule of aspartame, we can follow these steps:
Step 1: Understand the Structure of Aspartame
Aspartame is a dipeptide methyl ester, which consists of two amino acids (aspartic acid and phenylalanine) linked by a peptide bond. The molecular formula is C14H18N2O5.
Step 2: Draw the Molecular Structure
To find the number of pi bonds, we need to visualize or draw the structure of aspartame. The key functional groups to consider are:
- The carbonyl groups (C=O)
- The aromatic ring (which contains double bonds)
Step 3: Identify the Bonds
1. Carbonyl Groups: Each carbonyl group (C=O) contains one pi bond. Aspartame has two carbonyl groups.
2. Aromatic Ring: The benzene ring in aspartame has three double bonds, contributing three pi bonds.
Step 4: Count the Pi Bonds
Now, we can count the total number of pi bonds:
- From the carbonyl groups: 2 pi bonds (one from each C=O)
- From the aromatic ring: 3 pi bonds (one from each C=C in the ring)
Step 5: Calculate the Total
Adding these together gives:
- Total pi bonds = 2 (from carbonyls) + 3 (from the aromatic ring) = 5 pi bonds.
Conclusion
Thus, the total number of pi bonds in one molecule of aspartame is 5.
---
Aspartame is a dipeptide methyl ester, which consists of two amino acids (aspartic acid and phenylalanine) linked by a peptide bond. The molecular formula is C14H18N2O5.
To find the number of pi bonds, we need to visualize or draw the structure of aspartame. The key functional groups to consider are:
- The carbonyl groups (C=O)
- The aromatic ring (which contains double bonds)
1. Carbonyl Groups: Each carbonyl group (C=O) contains one pi bond. Aspartame has two carbonyl groups.
2. Aromatic Ring: The benzene ring in aspartame has three double bonds, contributing three pi bonds.
Now, we can count the total number of pi bonds:
- From the carbonyl groups: 2 pi bonds (one from each C=O)
- From the aromatic ring: 3 pi bonds (one from each C=C in the ring)
Adding these together gives:
- Total pi bonds = 2 (from carbonyls) + 3 (from the aromatic ring) = 5 pi bonds.
Thus, the total number of pi bonds in one molecule of aspartame is 5.
Topper's Solved these Questions
Similar Questions
The major product 'x' formed in the following reaction is:
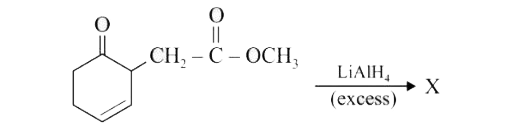
View SolutionFor following reactions
A400K−−→ Product
A200K−−−−−→catalyst Product
it was found that the Ea is decreased by 20 kJ/ mol in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is ( Assume per exponential factor is same ) :
View SolutionWhich one of the following molecules contains no pi - bond ?
View SolutionThe number of Oxygen atoms in one molecule of “Aspartame” is ________
View SolutionThe number of Pi bonds in one molecule of “Aspartame” is ________
View SolutionThe number of σ and π-bonds in a molecule of benzene is
View SolutionWhat is the number of σ and π-bonds in a molecule of ethyne?
View SolutionC2 अणु में σ और π बंधन की संख्या है
View SolutionWhich one of the following molecules contains no π bond ?
View SolutionWhich one of the following molecules contain no pi -bond ?
View Solutionno.of pi - bonds in one molecule of gammaxene is
View SolutionWhich one of the following molecules contains no π - bond ?
View SolutionThe number of Oxygen atoms in one molecule of “Aspartame” is ________
View SolutionWhich one of the following molecules contains no π - bond ?
View SolutionWhich one of the following molecules contains no π bond?
View Solution

