One mole of a monatomic gas is taken from a point A to another point B along the path ACB. The initial temperature at A is T0. Calculate the heat abosrbed by the gas in the process A→C→B.
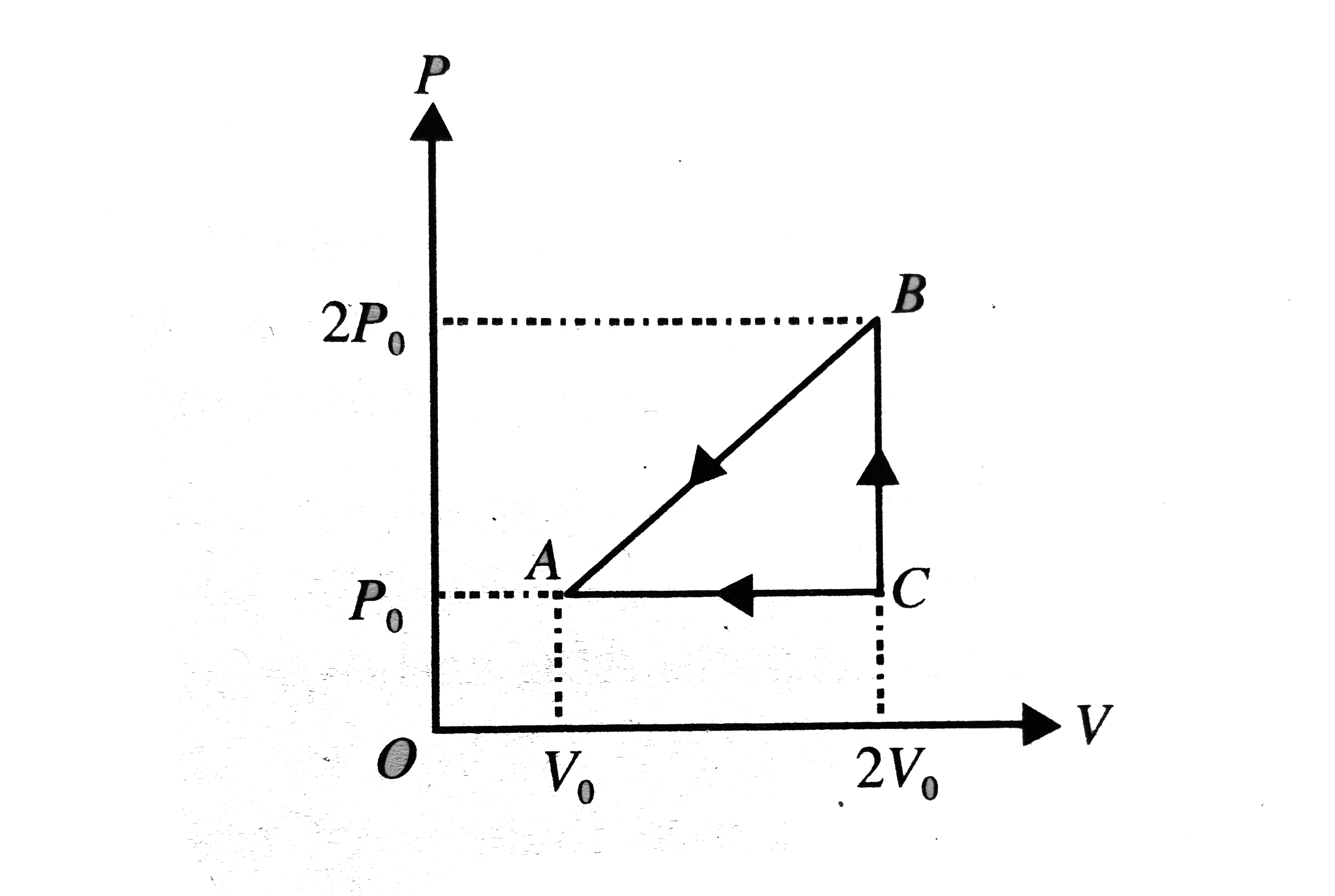
The correct Answer is:11
If TB be the temperature at B, then by gas law
PAVATB=PBTB ∴TB=PBVBPATATA=(2P0)(2V0)P0V0T0
The change in internal energy from A to B
ΔU=nCvΔT=11×3R2×(2V0−V0) =9RT02
Work done in the process A to C WAC=PΔV=P0(2V0−V0)
=P0V0=RT0 and WCB=0 ∴ Total work done form A→C→B
WAC+QCB=RT0+0=RT0 From first law of thermodynamics
Q=DetlaU=W =9RT02+RT0=11RT02
Thus heat absorbed by the gas from A→C→B is 11RT02
टॉपर्स ने हल किए ये सवाल
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:Level - 2
Explore 40 Videos
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:JEE MAIN (ARCHIVE )
Explore 81 Videos
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:level-0 Short Answer Type – II
Explore 24 Videos
- ENERGY & MOMENTUMBook:VMC MODULES ENGLISHChapter:ENERGY & MOMENTUMExercise:JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE
Explore 1 Video
- GRAVITATIONBook:VMC MODULES ENGLISHChapter:GRAVITATIONExercise:JEE Advance (Archive) TRUE/FALSE
Explore 1 Video
समान प्रश्न
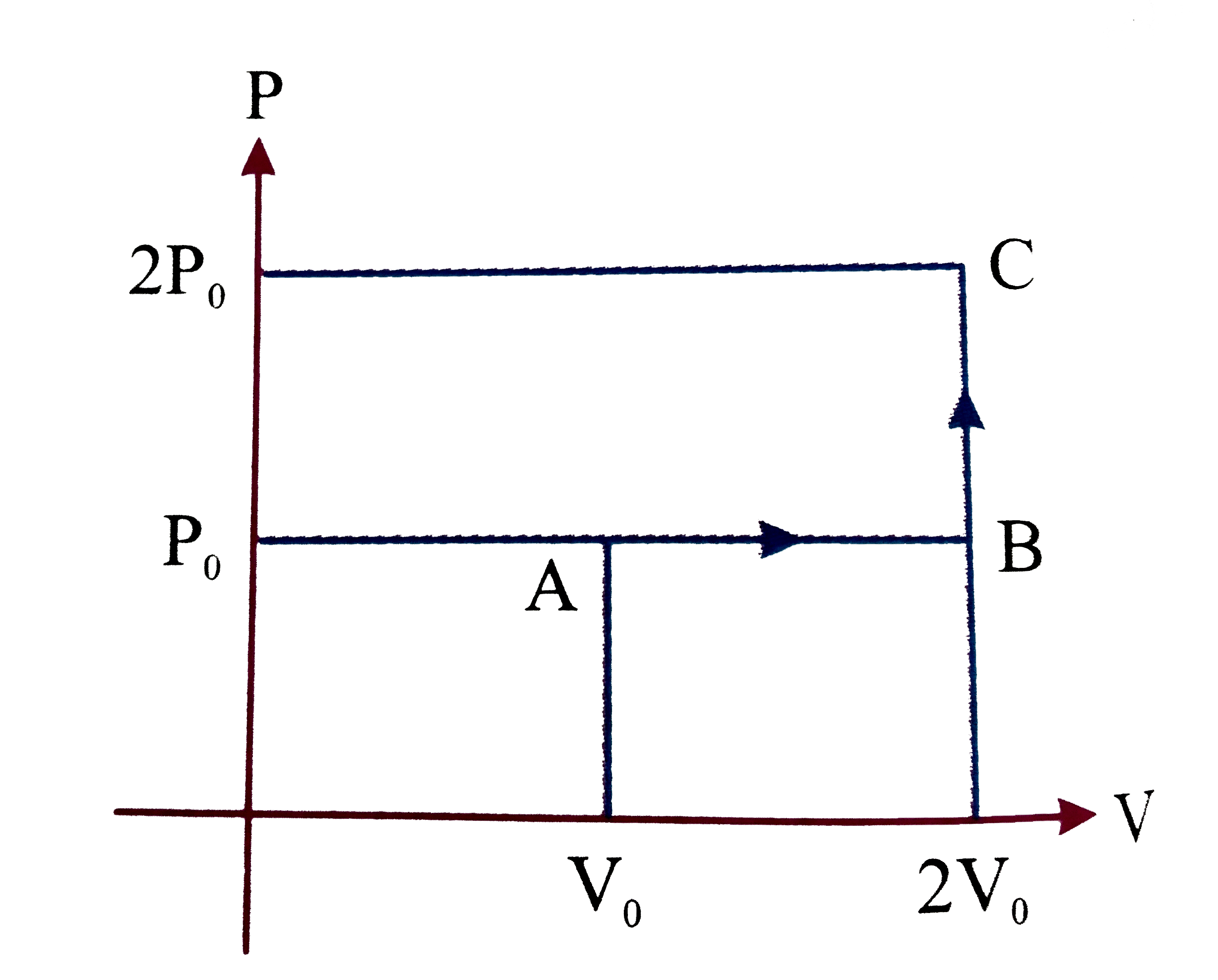
One mole of an ideal monoatomaic gas is taken from A to C along the path ABC . The temperature of the gas at A is T0 . For the process ABC :
View SolutionOne mole of ideal monoatomic gas is taken round the cyclic process as shown in figure.
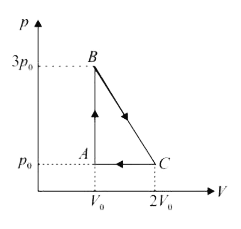
The heat rejected by the gas in the path CA is ………...and the heat absorbed by the gas in the path AB is ………...........
View SolutionOne mole of an ideal gas is taken from state A to state B by three different processes (a) ACB, (b) ADB and (c ) AEB as shown in the P−V diagram. The heat absorbed by the gas is
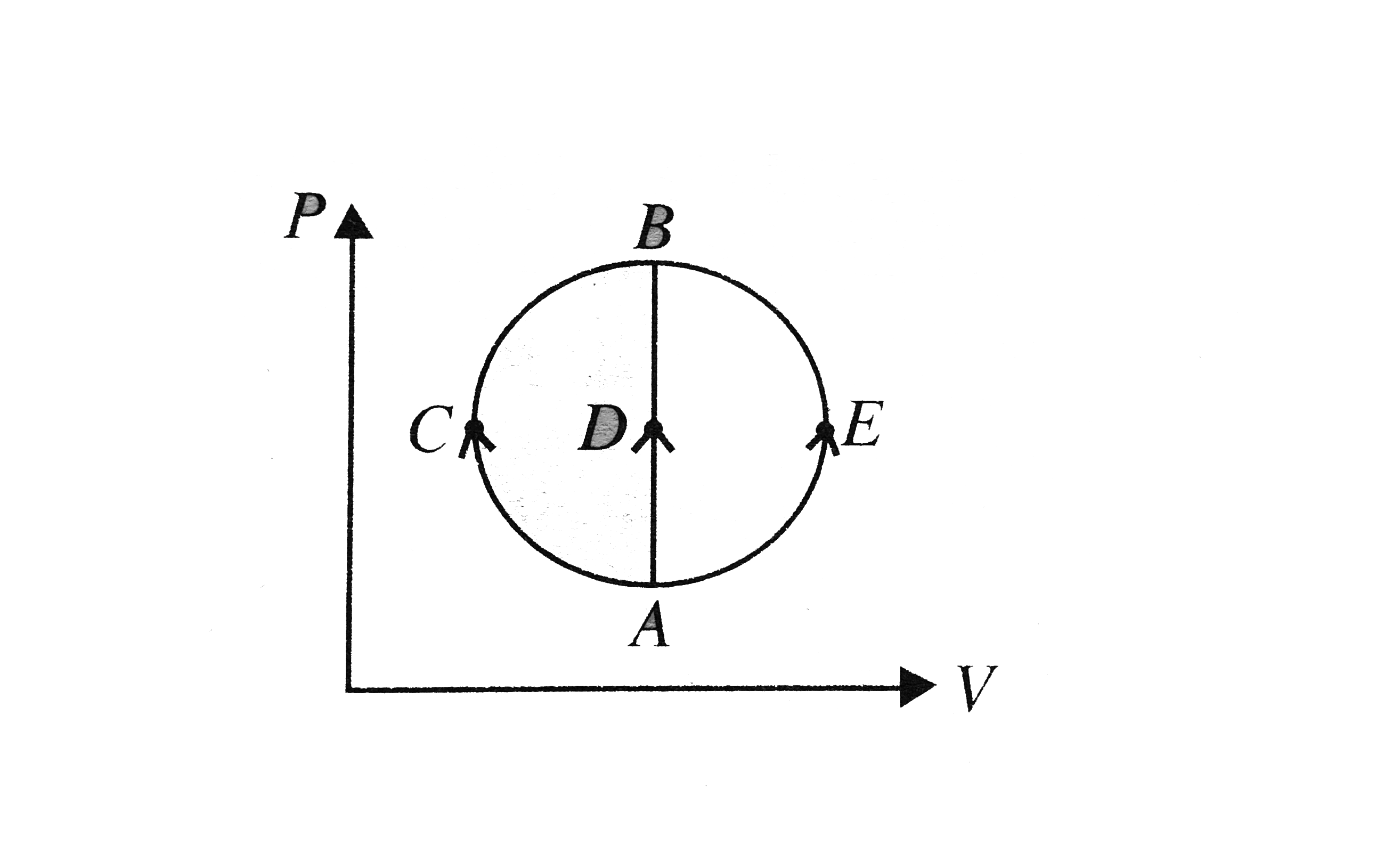
View SolutionOne mole of an ideal gas is taken from state A to state B by three different processes (a) ACB, (b) ADB and (c ) AEB as shown in the P−V diagram. The heat absorbed by the gas is

View SolutionOne mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by :

View SolutionOne mole of monoatomic gas is taken round the cyclic process. Heat absorbed by gas in process AB is
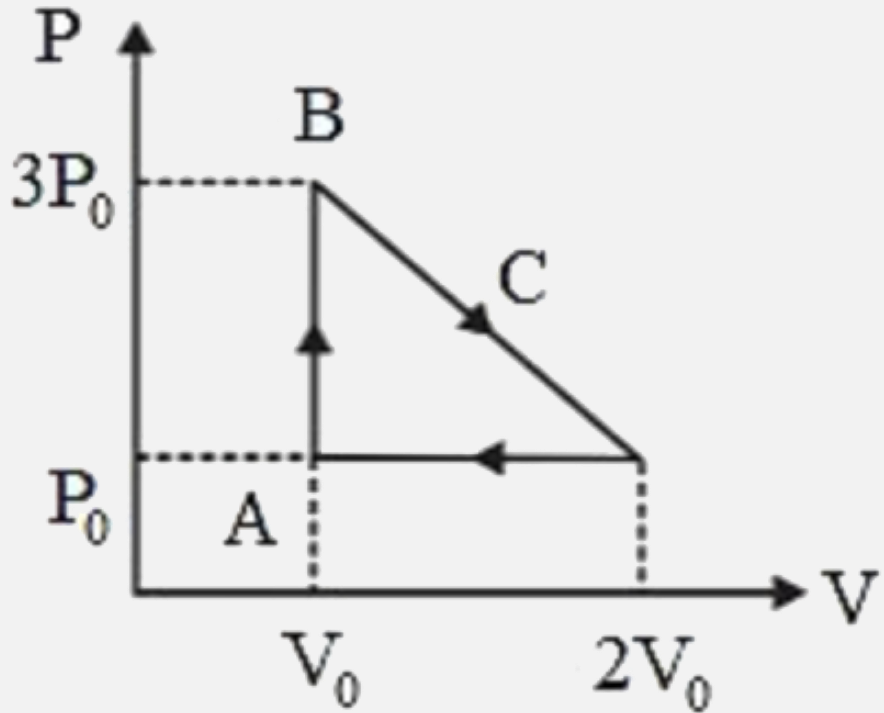
View SolutionOne mole of an ideal monatomic gas at temperature T0 expands slowly according to the law P=kV (k is constant). If the final temperature is 4T0 then heat supplied to gas is
View SolutionTwo moles of a monoatomic gas are taken from a to c, via three paths abc, ac and adc.
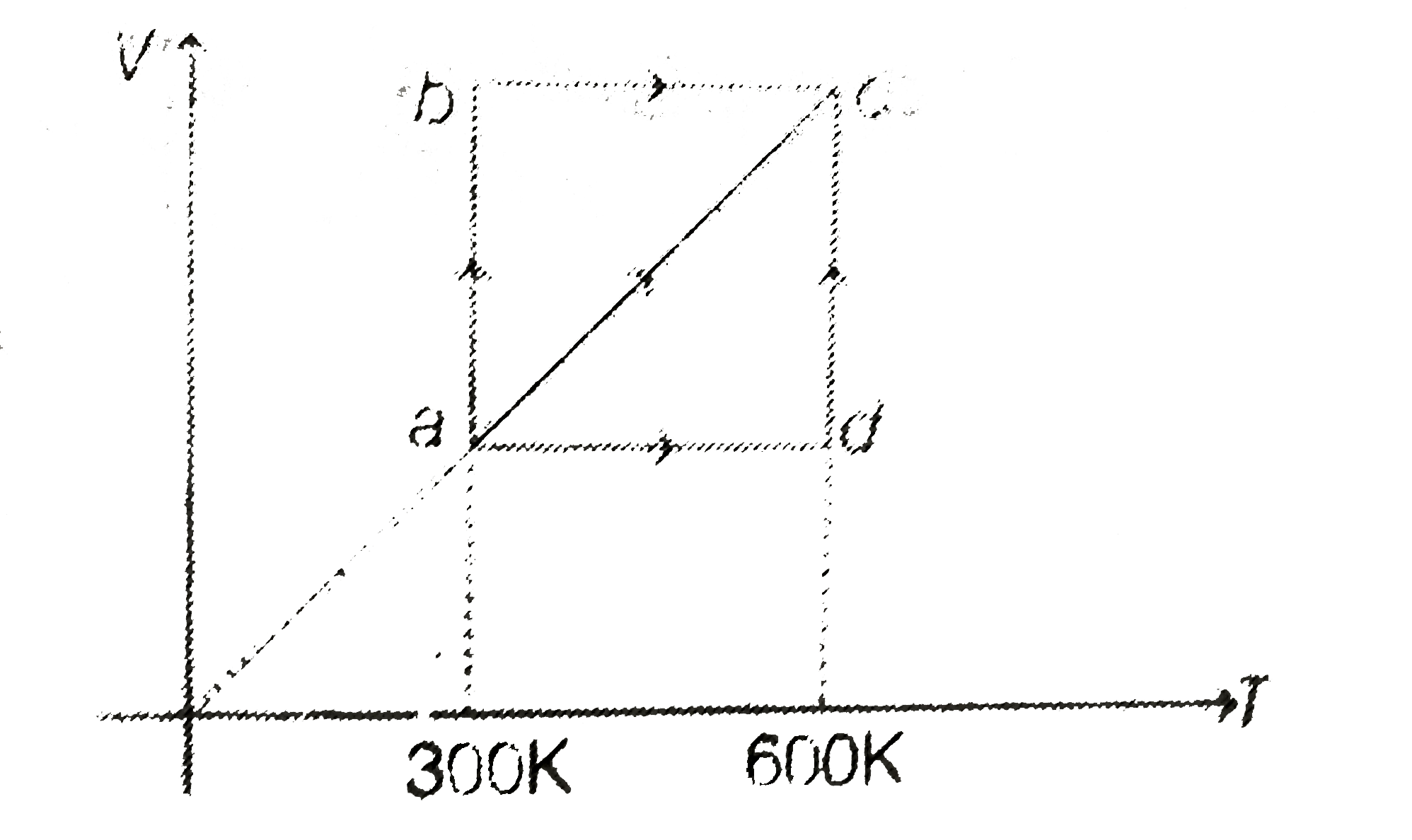
Work done by the gas in process ac is
View SolutionOne mole of an ideal monoatomic gas is taken through a polytropic process P2T = constant. The heat required to increase the temperature of the gas by ΔT is
View SolutionP-V curve of a diatomic gas is shown in the Fig. Find the total heat given to the gas in the process A→B→C
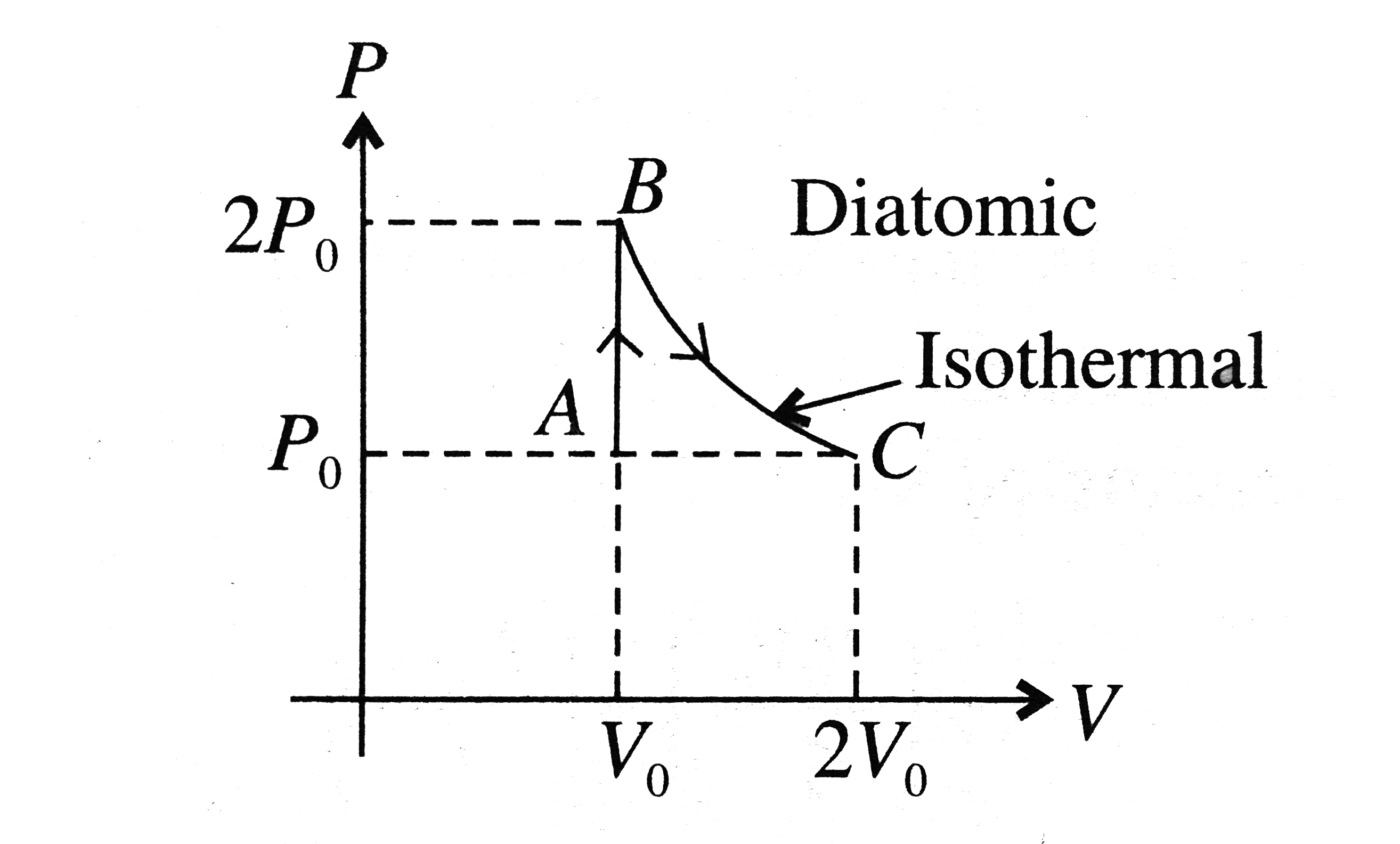
View Solution
