Which of the following has most acidic proton?
A
CH3COCH3
B
(CH3)2C=CH2
C
CH3COCH2COCH3
D
(CH3CH2)3CH
Text Solution
The correct Answer is:C
Proton of active methylene group is acidic in nature 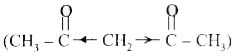 has two electron withdrawing groups around −CH2 - which make it active methylene group.
has two electron withdrawing groups around −CH2 - which make it active methylene group.
|
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:MTG GUIDEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:NEET CAFE (PROPERTIES OF CARBOXYLIC ACIDS AND THEIR DERIVATIVES )
Explore 50 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:MTG GUIDEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:NEET CAFE (USES OF ALDEHYDES, KETONES AND CARBOXYLIC ACIDS)
Explore 4 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:MTG GUIDEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:NEET CAFE (PREPARATION OF ALDEHYDES AND KETONES)
Explore 16 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:MTG GUIDEChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:AIPMT/NEET
Explore 16 Videos
- BIOMOLECULESBook:MTG GUIDEChapter:BIOMOLECULESExercise:AIPMT/NEET (MCQS)
Explore 19 Videos
Similar Questions
Knowledge Check
Which of the following is most acidic?
A(CH3)2CHOHB(CH3)3COHCCH3OHDCH3CH2OHWhich of the following is most acidic?
ACH3OHBC2H5OHCCH3CH2CH2OHDH2OWhich of the following is most acidic?
APhenolBBenzyl alcoholCm-ChlorophenolDCyclohexanol

