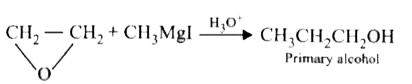Which one of the following will produce a primary alcohol by reacting with CH3MgI?
Ethylene oxide
Ethyl acetate
Methyl cyanide
Acetone
The correct Answer is:A
टॉपर्स ने हल किए ये सवाल
- ALCOHOLS, PHENOLS AND ETHERSBOOK - MTG GUIDECHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - CHECK YOUR NEET
21 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - MTG GUIDECHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - AIPMT/NEET
16 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - MTG GUIDECHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - AIPMT/NEET
16 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - MTG GUIDECHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - AIPMT/NEET
21 Videos
समान प्रश्न
Phenols are less reactive than alcohols in reactions involving C - O fission. This is due to
View SolutionGiven are cyclohexanol (I), acetic acid (II), 2,4,6-trinitrophenol (III) and phenol (IV). In these the order of decreasing acidic character will be
View SolutionGiven are cyclohexanol (I), acetic acid (II), 2,4,6-trinitrophenol (III) and phenol (IV). In these the order of decreasing acidic character will be
View SolutionWhich of the following reactions will not result in the formation of carbon-carbon bonds?
View SolutionWhich one of the following will produce a primary alcohol by reacting with CH_(3)MgI ?
View SolutionThe number of moles of CH_(4) formed by reacting 96 grams of CH_(3)OH with excess CH_(3)MgI is
View Solutionनिम्नलिखित में से कौन CH3MgI के साथ क्रिया करके प्राइमरी ऐल्कोहॉल बनाएगा ?
View SolutionCH3MgI की ऐसीटोन के साथ क्रिया से निर्मित उत्पाद के जल-अपघटन पर प्राप्त होता है-
View SolutionEthyl alcohol cannot be used as a solvent for CH_(3)MgI because
View SolutionHow does formaldehyde react with
CH3MgI followed by hydrolysis
View SolutionWhich one of the following compounds will be obtained when ethyl formate is allowed to react with excess of CH3MgI and the resulting compound is hydrolysed-
View SolutionWhen CH3MgI reacts with CH3CN and the product is hydrolysed, we get-
View SolutionWhich one of the following is a primary alcohol?
View SolutionEthyl alcohol cannot be used as a solvent for CH_(3)MgI because
View SolutionHow does formaldehyde react with (i) NH3, (ii) CH3MgI followed by hydrolysis and (iii) NaOH.
View SolutionEthyl alcohol cannot be used as a solvent for CH3MgI because
View Solution