0.2 molal aqueous solution of acid HX is 20% ionised. Kf=1.86 K molal−1. The freezing point of the solution is very close to:Solution in Malayalam
−0.45∘C
−0.90∘C
−0.31∘C
−0.53∘C
The correct Answer is:A
△Tf=Kr× molality ×(1+α)HA1−α⇔H+α+A−α
△Tr=1.86×0.2×1.2
△Tf=0.45 Tf=−0.45∘C
Topper's Solved these Questions
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level II
Explore 50 Videos
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level II (Assertion & Reason Type)
Explore 20 Videos
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level-III (Linked Comprehension Type)
Explore 12 Videos
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3 (Linked Comprehension Type)
Explore 10 Videos
- STEREOCHEMISTRY Book:BRILLIANT PUBLICATIONChapter:STEREOCHEMISTRY Exercise:LEVEL-II (ASSERTION - REASON TYPE)
Explore 2 Videos
Similar Questions
The volumes of two HCl solutions A (0.5N) and B(0.1N) to be mixed for preparing 2 L of 0.2 NHCl are:
View SolutionAssertion : If a liquid solute more volatile than the solvent is added to the solvent, the papour pressure of the solution may increase i.e. ps>p∘.
Reason In the presence of a more volatile liquid solute, only the solute will form the vapours and solvent will not.
View Solution1 mole of A and 1 mole B are mixed together to form an ideal solution at 25∘C. Solution is placed in a vessel fitted with piston. If pressure on the solution is reduced isothermally.
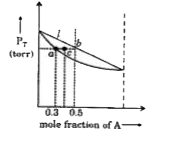
Correct relationship between P∘AandP∘B is
View SolutionF centre is an example of metal excess defect due to the presence of extra cations at interstitial sites. Zinc oxide is white in colour at room temperature, on heating it loses oxygen and turns yellow.
ZnOHeat−−→Zn2++12O2+2e−
Now there is excess of zinc in the crystal and its formula becomes Zn1+x O. the excess of Zn2+ ions move to interstitial sites and the electrons to neighbouring interstitial sites.
There are many solids which are difficult to prepare in the stiochiometric composition and contain less amount of the metal as compared to stiochiometric proportion. a typical example of this type is FeO which is mostly found with a composition of Fe0.95. it may actually range from Fe0.96 O.
Q. In Fe0.95 O:
View SolutionAssertion : Meso tartaric acid is optically inactive
Reason : Meso tartaric acid has a plane of symmetry
View SolutionA 0.2 molal aqueous solution of a weak acid HX is 20% ionized. The freezing point of the solution is (k_(f) = 1.86 K kg "mole"^(-1) for water):
View SolutionA 0.2 molal aqueous solution of a weak acid (HX) is 20 percent ionised. The freezing point of this solution is (Given Kf=1.86.∘CKgmol−1 for water):
View SolutionA 0.2 molal aqueous solution of weak acid (HX) is 20% ionised. The freezing point of this solution is (Given, Kf=1.86∘Cm−1 for water)
View SolutionA 0.2 molal aqueous solution of weak acid (HX) is 20% ionised. The freezing point of this solution is (Given, Kf=1.86∘Cm−1 for water)
View Solution0.2 molal acid HX is 20% ionised in solution, Kf=1.86Kmolality−1. The freezig point of the solution is
View Solution0.2 molal acid HX is 20% ionised in solution K_"f" = 1.86 K "molality"^(–1) . The freezing point of the solution is -
View SolutionIn a 2.0 molal aqueus solution of a weak acid HX the degree of disssociation is 0.25. The freezing point of the solution will be nearest to: (Kf=1.86Kkgmol−1)
View SolutionIf 0.2 molal aqueous solution of a weak acid (HA) is 40% ionised then the freezing point of the solution will be (Kf for water = 1.86∘C
View SolutionA 0.2 Molal aqueous solution of a weak acid (HX) is 20 % ionised . The freezing point of this solution is ______.
(Given =Kf=1.86∘Cm for water )
View SolutionA 0.2 molal aqueous solution of a weak acid (HX) is 20 percent ionised. The freezing point of this solution is: ( K_f = 1.86 K/m for water)
View SolutionA 0.2 molal aqueous solution of a weak acid (HX) is 20 percent ionised. The freezing point of this solution is: (Kf = 1.86 K/m for water)
View Solution
