which one of the following ions is not tetrahedral in shape ?
A
[NiCl4]2−
B
NH−4
C
BF−4
D
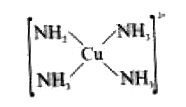
[Cu(NH3)4]2
Text Solution
The correct Answer is:D
[Cu(NH3)4]2+ is not tetrahedral . It is a square planar complex.
|
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:PHYSICS WALLAHChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:Level -2
Explore 48 Videos
- BIOMOLECULESBook:PHYSICS WALLAHChapter:BIOMOLECULESExercise:NEET PAST 5 YEARS QUESTIONS
Explore 13 Videos
- CHEMISTRY IN EVERDAY LIFE Book:PHYSICS WALLAHChapter:CHEMISTRY IN EVERDAY LIFE Exercise:NEET PAST 5 YEARS QUESTIONS
Explore 6 Videos
Similar Questions
Knowledge Check
Which of the following is not tetrahedral in shape ?
ANH+4BSiCl4CSF4DSO2−4Which of the following is not tetrahedral in shape ?
ANH+4BSiCl4CSF4DSO2−4Which of the following is not tetrahedral in shape ?
ANH+4BSiCl4CSF4DSO2−4

