Consider the molecules CH4,NH3 and H2O which of the given statement is false ?
The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3
The H-C-H bond angle in CH2 the H-C-H bond angle in NH3 and the H−O−H bond angle in H2O are all greater than 90∘
Then H-O-H bond angle in H2O is lager than the H-C-H bond angle in CH4
The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3
The correct Answer is:C
CH4−sp3 hybridised, tetrahedral bond angle 109∘28.

NH,−sp3 hybridised, bond angle - 107∘
H2O−sp3 bend shaped
bond angle −104∘5.
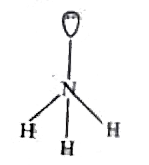
So , bond angle of H2O is less than that NH3&CH4
टॉपर्स ने हल किए ये सवाल
- CHEMICAL BONDING AND MOLECULAR STRUCTURE BOOK - PHYSICS WALLAHCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTURE EXERCISE - Level -2
48 Videos
- BIOMOLECULESBOOK - PHYSICS WALLAHCHAPTER - BIOMOLECULESEXERCISE - NEET PAST 5 YEARS QUESTIONS
13 Videos
- CHEMISTRY IN EVERDAY LIFE BOOK - PHYSICS WALLAHCHAPTER - CHEMISTRY IN EVERDAY LIFE EXERCISE - NEET PAST 5 YEARS QUESTIONS
6 Videos
समान प्रश्न
An ionic compound A+B− is most likely to be formed when :
View SolutionWhich of the following is a basic amino acid?
View SolutionWhich of the following is a cationic detergent?
View SolutionConsider the molecules CH4,NH3 and H2O which of the given statement is false ?
View SolutionArrange H2O,NH3 and CH4 molecules in decreasing order of bond angle.
View SolutionConsider the molecules CH_(4), NH_(3) and H_(2) O Which of the given statements is false ?
View SolutionConsider the molecules CH_(4),NH_(3) and H_(2)O . Which of the given statements is false-
View SolutionConsider the molecules CH4,NH3 and H2O . Which of the given statements is false ?
View SolutionThe bond angles in molecules H_(2)O, NH_(3), CH_(4) and CO_(2) are in the order :
View SolutionThe bond angles in molecules H_(2)O, NH_(3), CH_(4) and CO_(2) are in the order :
View SolutionH_(2)O, NH_(3), CH_(4) are the examples of homoatomic molecules.
View SolutionDiscuss the shapes of following molecules : NH_(3), H_(2)O, CH_(4), PCl_(5) and SO_(2) .
View SolutionConsider the molecules CH4,NH3 and H2O Which of the statements is false?
View SolutionThe decreasing order of alkaline strength is: (I) CH_(3)NH_(2) (II) C_(6)H_(5)CH_(2)NH_(2) (III) p-CH_(3)O-C_(6)H_(4)-NH_(2) (IV) m-CH_(3)O -C_(6)H_(4) - NH_(2)
View SolutionDiscuss the shapes of following molecules : NH_(3), H_(2)O, CH_(4), PCl_(5) and SO_(2) .
View SolutionDiscuss the shapes of following molecules : NH_(3), H_(2)O, CH_(4), PCl_(5) and SO_(2) .
View Solution

