Acetophenone can be prepared by Friedel crafts reaction between benzene and
Benzoyl chloride
Benzyl chloride
Ethanoyl chloride
Methanoyl chloride
The correct Answer is:C
To prepare acetophenone via the Friedel-Crafts reaction, we need to identify the appropriate reactant that will provide the necessary carbonyl and methyl groups. Here’s a step-by-step solution:
Step 1: Understand the structure of acetophenone
Acetophenone has the structure C6H5C(O)CH3. This indicates that it consists of a phenyl group (C6H5) attached to a carbonyl group (C=O), which is further attached to a methyl group (CH3).
Step 2: Identify the required reactants
In a Friedel-Crafts reaction, we typically react an aromatic compound (in this case, benzene) with an acyl halide to introduce an acyl group. The acyl group in acetophenone is derived from acetyl chloride (CH3C(O)Cl).
Step 3: Write the reaction
The reaction can be summarized as follows:
1. Benzene (C6H6) reacts with acetyl chloride (CH3C(O)Cl) in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3).
2. The reaction produces acetophenone (C6H5C(O)CH3) and hydrochloric acid (HCl) as a byproduct.
Step 4: Conclusion
Thus, the correct answer is that acetophenone can be prepared by the Friedel-Crafts reaction between benzene and acetyl chloride.
Final Answer:
Acetophenone can be prepared by Friedel-Crafts reaction between benzene and acetyl chloride (CH3C(O)Cl).
---
Acetophenone has the structure C6H5C(O)CH3. This indicates that it consists of a phenyl group (C6H5) attached to a carbonyl group (C=O), which is further attached to a methyl group (CH3).
In a Friedel-Crafts reaction, we typically react an aromatic compound (in this case, benzene) with an acyl halide to introduce an acyl group. The acyl group in acetophenone is derived from acetyl chloride (CH3C(O)Cl).
The reaction can be summarized as follows:
1. Benzene (C6H6) reacts with acetyl chloride (CH3C(O)Cl) in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3).
2. The reaction produces acetophenone (C6H5C(O)CH3) and hydrochloric acid (HCl) as a byproduct.
Thus, the correct answer is that acetophenone can be prepared by the Friedel-Crafts reaction between benzene and acetyl chloride.
Acetophenone can be prepared by Friedel-Crafts reaction between benzene and acetyl chloride (CH3C(O)Cl).
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACID BOOK - PHYSICS WALLAHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACID EXERCISE - NEET PAST 5 YEARS QUESTIONS
16 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACID BOOK - PHYSICS WALLAHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACID EXERCISE - NEET PAST 5 YEARS QUESTIONS
16 Videos
- ALCOHOLS, PHENOLS AND ETHERS BOOK - PHYSICS WALLAHCHAPTER - ALCOHOLS, PHENOLS AND ETHERS EXERCISE - NEET PAST 5 YEARS QUESTIONS
26 Videos
- AMINES BOOK - PHYSICS WALLAHCHAPTER - AMINES EXERCISE - NEET Past 5 Years Questions
11 Videos
Similar Questions
Knowledge Check
Benzophenone can be obtained by Friedel Crafts reaction beteween benzene and
ABenzyl chlorideBChlorobenzeneCBenzoyl chorideDNone of theseThe compound shows below
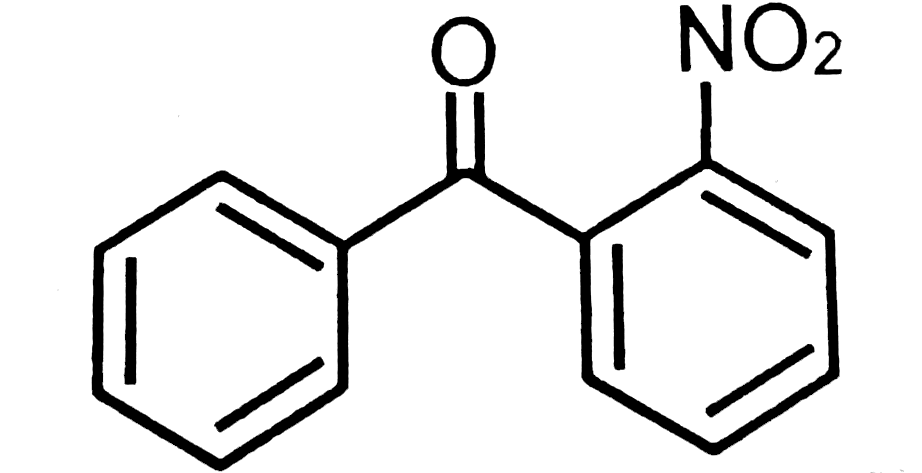
can be readily prepared by Friedel-Crafts reaction betweenAbenzene and 2-nitrobenzoyl chlorideBbenzyl chloride and nitrobenzeneCnitrobenzene and benzoyl chlorideDbenzene and 2-nitrobenzyl chlorideWhich of the following can be used in Friedel Crafts reaction when benzene used with AlCl3
A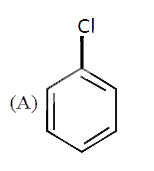 BCH2=CH–ClCCH3CH2ClDCH2=CH–CH2–Cl
BCH2=CH–ClCCH3CH2ClDCH2=CH–CH2–Cl
Friedel-Crafts reaction is not given by:
View SolutionIn Friedel-Craft's reaction, toulene can be prepared by
View SolutionAssertion : Alkyl benzene is not prepared by Friedel− Crafts alkylation of benzene.
Reason : Alkyl halides are more reactive than acyl halides.
View SolutionAssertion : Alkyl benzene is not prepared by Friedel Craft alkylation of benzene.
Reason : Grignard reagents react with hydroxyle group
View SolutionAssertion : Alkyl benzene is not prepared by Friedel− Crafts alkylation of benzene.
Reason : Alkyl halides are more reactive than acyl halides.
View Solution

