Heat of combustion of methane at constant volume and 298 K has been found to be −885.0kJmol−1. Calculate the enthalpy of combustion under constant pressure. (R=8.314Jmol−K−1).Solution in Malayalam
The combustion of methane is
CH4(g)+2O2(g)→CO2(g)+2H2O(l)
Hence, Δng=1mol ( gaseques product) -2 moles (gaseous reactants) =−2 mols.
Change in enthalpy, ΔH=ΔU+ΔngRT
ΔH=−885000Jmol−1+(−2mol)(8.314JK−1mol−1)(298K)
ΔH=−885000Jmol−1−4955mol−1=−889955Jmol−1=−889.955kJmol−1
Topper's Solved these Questions
- CHEMICAL THERMODYNAMICS BOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL THERMODYNAMICS EXERCISE - LEVEL-I
92 Videos
- CHEMICAL THERMODYNAMICS BOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL THERMODYNAMICS EXERCISE - LEVEL-II
102 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - BRILLIANT PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - QUESTION (LEVEL -ll)
42 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBOOK - BRILLIANT PUBLICATIONCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESEXERCISE - QUESTIONS (LEVEL-III ( Linked Comprehension Type ))
12 Videos
Similar Questions
In ....... process, work is done at the expense of internal energy.
View SolutionWhich of the following statements is correct?
View SolutionWhich of the following species are hypervalent ?
(l) ClO−4
(ll) BF3
(lll) SO2−4
(lV) CO2−3
View SolutionThe heats of formation (ΔH∘f) of the oxides of the third period, sodium to chlorine, are in kJ mol−1
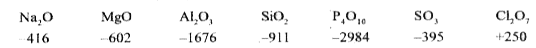
Based on these data, answer the following questions
Which oxide has maximum negative heat of formation per oxygen atom?
View SolutionHeat of combustion of ethanol at constant pressure and at temperature T K(=298 K) is found to be -qJ "mol"^(-1) . Hence , heat of combustion (in J "mol"^(-1) ) of ehtanol at the same temperature at constant volume will be:
View SolutionThe heat of combustion of napthalene {C_(10)H_(8)(s)} at constant volume was measured to be -5133 kJ mol^(-1) at 298 K. Calculate the value of enthalpy change (Given R=8.314 JK^(-1)mol^(-1) )
View SolutionThe combustion of benzene (l) gives CO_(2) (g) and H_(2)O (l) . Given that heat of combustion of benzene at constant volume is -3263.9 kJ * mol^(-1) at 25^(@) C , heat of combustion ( in kJ * mol^(-1)) of benzene at constant pressure will be - (R = 8.314 J * K^(-1) * mol^(-1) )
View SolutionCalculate the enthalpy of combustion of ethylene at 300K at constant pressure if its enthalpy of combustion at constant volume is −1406 kJ mol−1.
View SolutionIn a constant volume calorimeter, 3.5 g of a gas with molecular weight 28 was burnt in ress oxygen at 298 K. The temperature of the calorimeter was found to Increase from 298 K to 298.45 K due to the combustion process. Given that the calorimeter constant is 2.5 KJ K^(-1) .Calculate the enthalpy of combustion of the gas in "kJ mol"^(-1) .
View SolutionThe heat of combustion of gaseous methan (CH_(4)) at constant volume is measured in a bomb calorimeter at 298 K and is found to be -885.4 kJ mol^(-1) .Find the value of enthalpy change.
View SolutionCalculate the enthalpy of combustion of ethylene at 300K at constant pressure if its enthalpy of combustion at constant volume is −1406 kJ mol−1.
View SolutionCalculate the enthalpy of combustion of ethylene at 300K at constant pressure if its enthalpy of combustion at constant volume is −1406 kJ mol−1.
View SolutionCalculate the enthalpy of combustion of ethylene at 300K at constant pressure if its enthalpy of combustion at constant volume is −1406 kJ mol−1.
View SolutionCalculate the enthalpy of combustion of ethylene at 300K at constant pressure if its enthalpy of combustion at constant volume is -"1406 kJ mol"^(-1) .
View SolutionIf the heat of combustion of carbon monoxide at constant volume and at 17^@ C is -283.3 kJ, then its heat of combustion at constant pressure is _________ . (R = 8.314 J K^-1 mol^-1 )
View Solution

