Assertion : A simplified coordination number ratio for ions of any ionic crystal is inverse of molar ratio.
Reason : Coordination number ratio of cation to anion in CaF2 (fluorite structure) is 8 : 4.Solution in Malayalam
If both (A) and ( R) are correct and ( R) is the correct explanation of(A).
If both (A) and ( R) are correct, but ( R) is not the correct explanation of (A).
If (A) is correct, but ( R) is incorrect.
If both (A) and ( R) are incorrect.
The correct Answer is:B
Step by step video & text solution for Assertion : A simplified coordination number ratio for ions of any ionic crystal is inverse of molar ratio. Reason : Coordination number ratio of cation to anion in CaF_(2) (fluorite structure) is 8 : 4. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLID STATEBOOK - BRILLIANT PUBLICATIONCHAPTER - SOLID STATEEXERCISE - QUESTIONS
14 Videos
- SOLID STATEBOOK - BRILLIANT PUBLICATIONCHAPTER - SOLID STATEEXERCISE - LEVEL-1
50 Videos
- SOLID STATEBOOK - BRILLIANT PUBLICATIONCHAPTER - SOLID STATEEXERCISE - LEVEL-II
50 Videos
- REDOX REACTION & ELECTROCHEMISTRYBOOK - BRILLIANT PUBLICATIONCHAPTER - REDOX REACTION & ELECTROCHEMISTRYEXERCISE - QUESTION (ELECTROCHEMISTRY) (LEVEL -II) (ASSERTION-REASON)
3 Videos
- SOLUTIONSBOOK - BRILLIANT PUBLICATIONCHAPTER - SOLUTIONSEXERCISE - Level-III (Linked Comprehension Type)
12 Videos
Similar Questions
A compound of elements X and Y crystallizes in cubic structure where X atoms occupy the corners of the cube and Y atoms occupy the centre of the cube. What is the formula of the compound?
View SolutionThe radius of a di val ent cation M2+ is 94 pm and of divalent anion X2− is 146 pm. Thus, M2X2− has
View SolutionTotal number of voids in 0.5 mole of a compound forming hexagonal closed packed structure are
View SolutionAssertion: After the electrolysis of aqueous CuSO4 solution pH decreases.
Reason: Sulphuric acid is formed.
View Solution1 mole of A and 1 mole B are mixed together to form an ideal solution at 25∘C. Solution is placed in a vessel fitted with piston. If pressure on the solution is reduced isothermally.
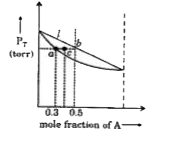
Correct relationship between P∘AandP∘B is
View SolutionFor some crystals , the radius ratio for cation and anion is 0.525 its coordination number will be
View SolutionAssertion:- Antigluorite structure is 4:8 (cation:anion) type coordination crystal.
Reason:- Ratio of no. of cation and anions in CaF2 crystal is 1:2
View SolutionThe coordination number of cation and anion in fluorite CaF2 and anti-fluroite Na2O are respectively:
View SolutionThe ratio of anion radius to cation radius of a crystal is 10:9,3. then , the coordination number of the cation in the crystal is
View SolutionIF the radius ratio of cation to anion is in the range of 0.225 - 0.414 , then the coordination number of cation will be _________.
View SolutionThe ratio of anion radius to cation radius of a crystal is 10 : 9.3 . The coordination number of the cation in the crystal is
View SolutionIn the fluorite structure, the coordination number of cation is:
View SolutionThe ratio of cationic radius to anionic radius in an ionic crystal is greater than 0.732. its coordination number is
View Solutionएक आयनिक क्रिस्टल में धनायनिक तथा ऋणायनिक त्रिज्या का अनुपात 0.414 से अधिक है, तो इसकी समन्वय संख्या है
View SolutionThe ratio of anion radius to cation radius of a crystal is 10 : 9.3. Then, the coordination number of the cation in the crystal is
View SolutionThe ratio of cationic radius to anionic radius in an ionic crystals is greater than 0.732. Its coordination number is
View Solution

