Which of the following properties is not shown by NO? Solution in Malayalam
Its bond order is 2.5
It is diamagnetic in the gaseous state
It is a neutral oxide
It combines with oxygen to form nitrogen dioxide
The correct Answer is:B
NO exists as a monomer in the gaseous state. Therefore, it has odd number of electrons (7+8=15) and hence is paramagnetic. All other statements are correct.
টপার এই প্রশ্নগুলো সমাধান করেছে
- P-BLOCK ELEMENTSBOOK - BRILLIANT PUBLICATIONCHAPTER - P-BLOCK ELEMENTSEXERCISE - LEVEL II
50 Videos
- P-BLOCK ELEMENTSBOOK - BRILLIANT PUBLICATIONCHAPTER - P-BLOCK ELEMENTSEXERCISE - LEVEL II (ASSERTION AND REASON)
20 Videos
- NUCLEAR CHEMISTRY BOOK - BRILLIANT PUBLICATIONCHAPTER - NUCLEAR CHEMISTRY EXERCISE - LEVEL-III (Linked Comprehension Type)
11 Videos
- POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE BOOK - BRILLIANT PUBLICATIONCHAPTER - POLYMERS, BIOMOLECULES AND CHEMISTRY IN EVERYDAY LIFE EXERCISE - LEVEL -III (Comprehension Type)
12 Videos
সম্পর্কিত প্রশ্ন
What may be expected to happen when phosphine gas is mixed with chlorine gas?
View SolutionAssertion : Salts of ClO−3 and ClO−4 are well known but those of FO−3 and FO−4 are non-existent.
Reason : F is more electronegative than O while Cl is less electronegative than O.
View SolutionA sample contains 10−2 kg of two substances A and B with half lives of 4 and 8 sec repsectively (Given that atomic mass of B is twice of A ).
The mass of A and B left after 16 second is :
View SolutionEvery amino acid has a carboxyl group and an amino group, and each group can exist in an acidic form or a basic form depending on the pH of the solution in which the amino acid is dissolved. The carboxyl groups of the amino acids have pKa values of approximately 2, the protonated amino group have pKa values near 9. Therefore, in a very acidic solution (pH near zero), both groups will be in their acidic forms. At a pH of 7, (which is greater than the pKa of the protonated amino group), the carboxyl group will be in its basic form and the amino group will be in its acidic form. In a strongly basic solution (say pH=11), both groups will be in the basic form.
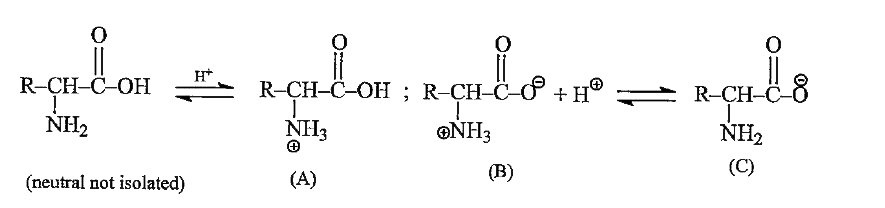
Form A(cationic) of an amino acid exists in
View SolutionWhich one of the following properties is not shown by NO ? .
View SolutionWhich of the following properties is not shown by NO ?
View SolutionWhich of the following properties is not shown by NO ?
View SolutionWhich of the following is a property shown by a magnet ?
View SolutionWhich of the following properties is not shown by NO ?
View SolutionWhich one of the following properties is not shown by NO?
View SolutionWhich of the following properties is not shown by NO ?
View SolutionWhich of the following properties is not shown by NO -
View SolutionWhich one of the following properties is not shown by NO?
View SolutionWhich one of the following properties is not shown by NO ? .
View SolutionWhich one of the following properties is not shown by NO ?
View Solution

