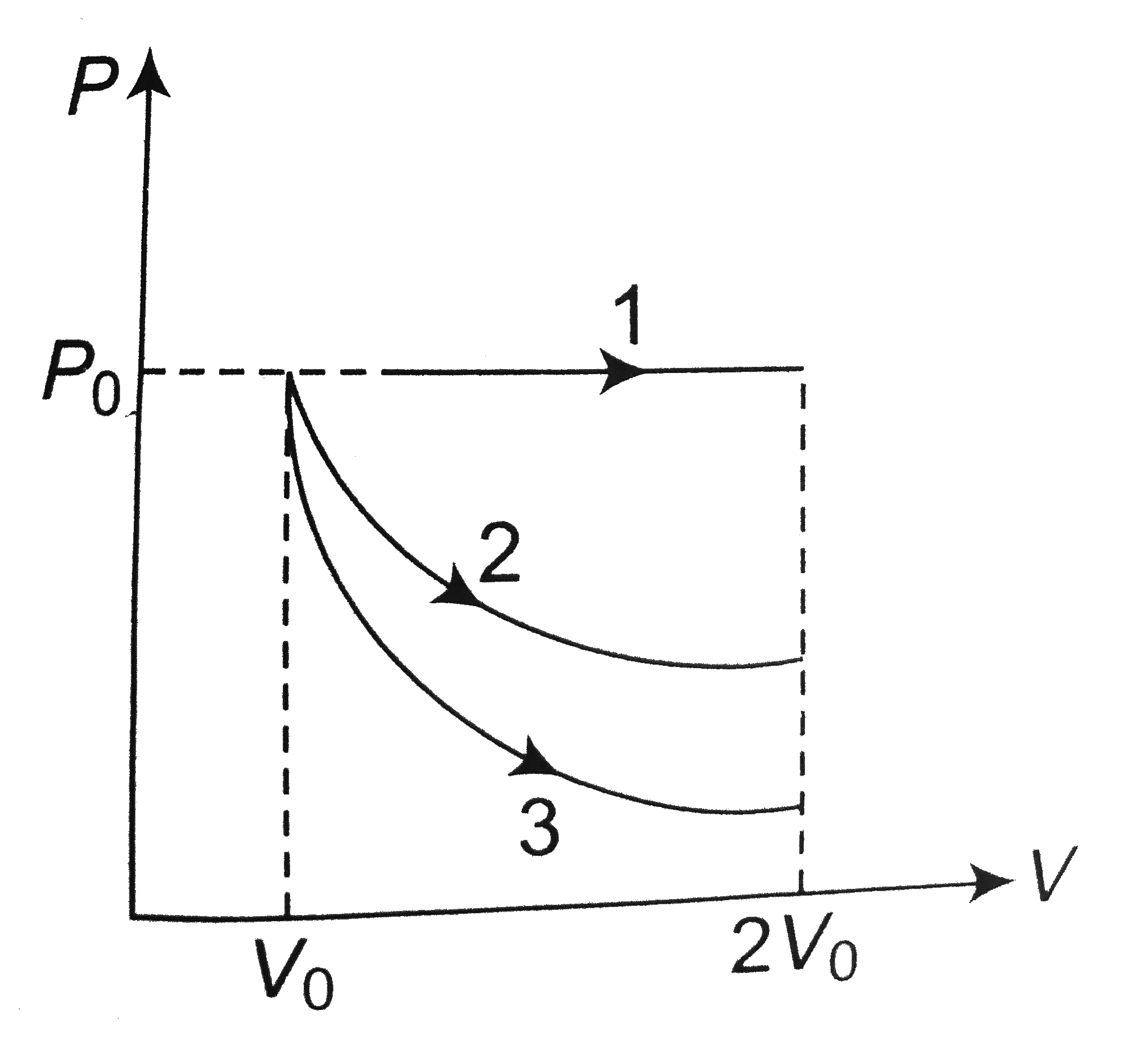
A gas is expanded from volume V0=2V0 under three different processes. Process 1 is isobaric process, process 2 is isothermal and process 3 is adiabatic. Let ΔU1,ΔU2andΔU3 be the change in internal energy of the gas in these three processes. then
 Solution in Gujrati
Solution in Gujrati
ΔU1>ΔU2>ΔU3
ΔU1<ΔU2<ΔU3
ΔU2<ΔU1<ΔU3
ΔU2<ΔU3<ΔU1
The correct Answer is:A
ΔU1=+ve
ΔU2=0
ΔU3=−ve
∴ΔU1>ΔU2>ΔU3
As volume increases , ΔW=+ve.
Topper's Solved these Questions
- GEOMETRICAL OPTICSBOOK - ALLEN CHAPTER - GEOMETRICAL OPTICSEXERCISE - EXERCISE -02
82 Videos
- GEOMETRICAL OPTICSBOOK - ALLEN CHAPTER - GEOMETRICAL OPTICSEXERCISE - EXERCISE -03
11 Videos
- GEOMETRICAL OPTICSBOOK - ALLEN CHAPTER - GEOMETRICAL OPTICSEXERCISE - SOME WORKED OUT EXAMPLES
83 Videos
- CURRENT ELECTRICITYBOOK - ALLEN CHAPTER - CURRENT ELECTRICITYEXERCISE - EX.II
66 Videos
- GRAVITATIONBOOK - ALLEN CHAPTER - GRAVITATIONEXERCISE - EXERCISE 4
9 Videos
Similar Questions
A triangular plate has two cavities , one square and one rectangular as shown in figure . The plate is heated.
_E01_066_Q01.png)
View SolutionThree liquids A,B and C having same specific heat and mass m , 2m and 3 m have temperature 20∘C,40∘C and 60∘C respectively. Temperature of the mixture when:
Column-IColumn-II(A)A and B are mixed(p)33∘C(B)A and C are mixed(q)52∘C(C)B and C are mixed(r)50∘C(D)A,B and C all three are mixed(s)46∘C(t)None
View SolutionIn an 20 m deep lake, the bottom is at a constant temperature of 4∘C. The air temperature is constant at −10∘C. The thermal conductivity of ice in 4 times that water. Neglecting the expansion of water on freezing, the maximum thickness of ice will be
View SolutionIn the Bohr's model of hy drogen atom, the electron moves around the nucleus in a circular orbit of radius 5×10−11m. Its time period is 1.5×10−16s. The current associated with the electron motion is:-
View SolutionAssertion : The difference in the value of acceleration due to gravity at pole and equator is proportional to square of angular velocity of earth.
Reason : the value of acceleration due to gravity is minimum at the equator and maximum at the pole.
View SolutionRefer to let DeltaU_(1) and DeltaU_(2) be the changes in internal energy of the system in the processes A and B. then
View SolutionRefer to let DeltaU_(1) and DeltaU_(2) be the changes in internal energy in the system in process A+B and DeltaW be the net work done by the system in the process A+B,
View SolutionAn ideal gas goes from State A to state B via three different process as indicate in the P-V diagram. If Q_(2), Q_(3) indicates the heat absorbed by the gas along the three processes and DeltaU_(1), DeltaU_(2), DeltaU_(3) indicates the change in internal energy along the three processes respectively, then
View SolutionA gas is expanded form volume V_(0) to 2V_(0) under three different processes as shown in the figure . Process 1 is isobaric process process 2 is isothermal and and process 3 is adiabatic . Let DeltaU_(1),DeltaU_(2) and DeltaU_(3) be the change in internal energy of the gs in these three processes then
View Solutionदिखाए गए P-V आरेख के अनुसार एक आदर्श गैस को तीन विभिन्न प्रक्रमों द्वारा स्थिति A से स्थिति B तक ले जाया जाता है | यदि इन तीन प्रक्रमों में अवशोषित ऊष्मा क्रमशः Q1,Q2 तथाQ3 है और आंतरिक ऊर्जा में परिवर्तन क्रमशः ΔU1,ΔU2 तथा ΔU3हो तो -
View SolutionThe initial state of 1 mol of an ideal gas is (P_(1),V_(1),T_(1)) . The gas is expanded by a reversible isothermal process and also by a reversible adiabatic process separately. If final volume of the gas is same in both the processes, and changes in internal energy in the isothermal and adiabatic processes are DeltaU_(1) and DeltaU_(2) respectively, then-
View SolutionA gas is expanded from volume V0 to 2V0 under three different processes, as shown in the figure. Process 1 is isobaric process, process 2 is isothermal and process 3 is adiabatic. Let ΔU1,ΔU2 and ΔU3 be the change in internal energy of the gas in these three processes. Then
View SolutionRefer to figure. Let ΔU1 and ΔU2 be the changes in internal energy of the system in the processes A and B. then
View SolutionRefer to figure. Let ΔU1 and ΔU2 be the changes in internal energy in the system in process A+B and ΔW be the net work done by the system in the process A+B,
View SolutionA body process 1 changes from stage A to stage B by process 2, as pictured. If the internal energy changes in process 1 DeltaU_(1) And internal energy changes in process 2 DeltaU_(2) Is then
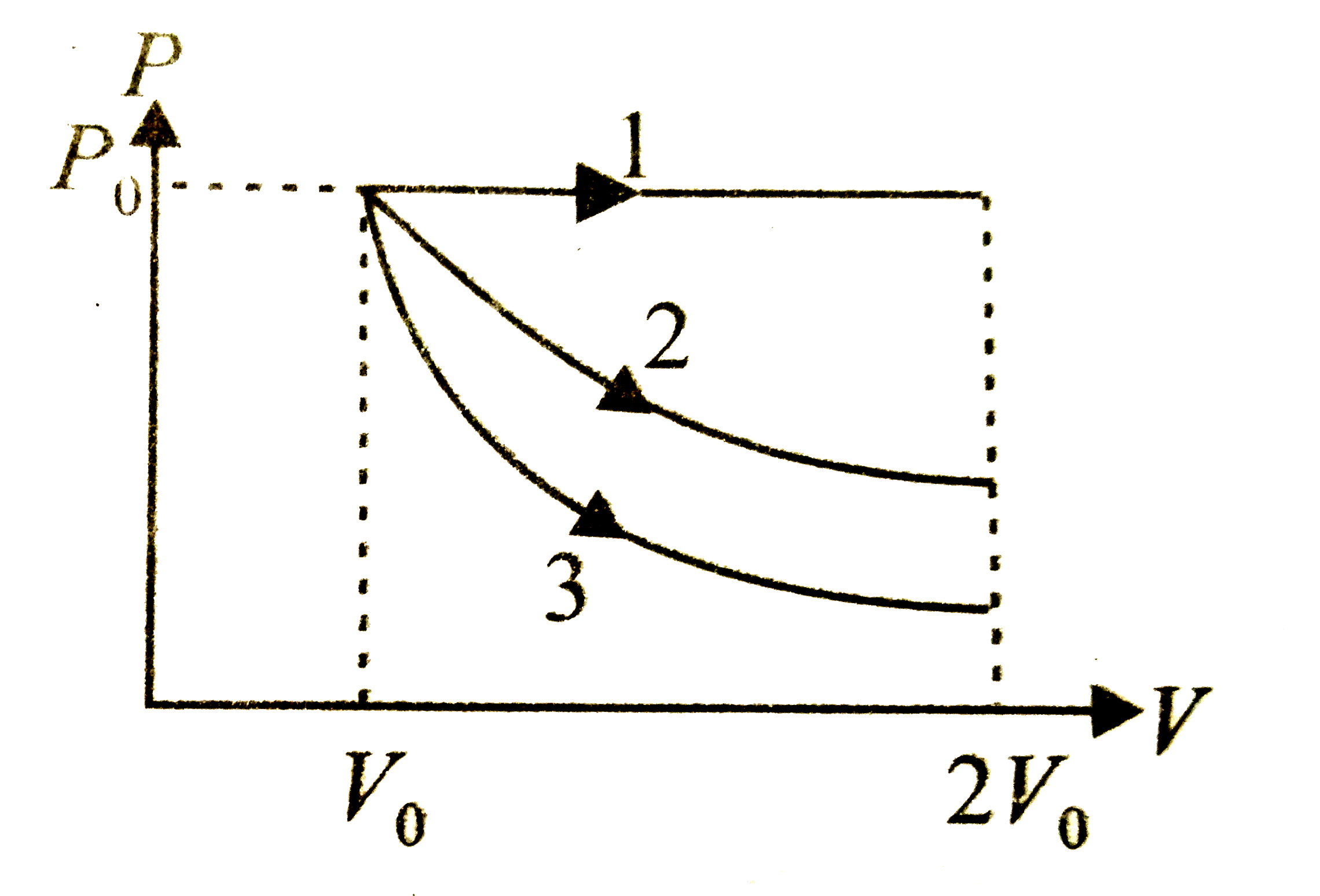
View SolutionA gas is expanded form volume V0→2V0 under three different processes as shown in the figure . Process 1 is isobaric process process 2 is isothermal and and process 3 is adiabatic .
Let ΔU1,ΔU2andΔU3 be the change in internal energy of the gas in these three processes then
View Solution

