Consider the figure of a defective crystal :
(a) Name the defect shown by this diagram.
(b) What is the effect of this defect on the solid ?
(c) Name as ionic compound which can show this type of defect.
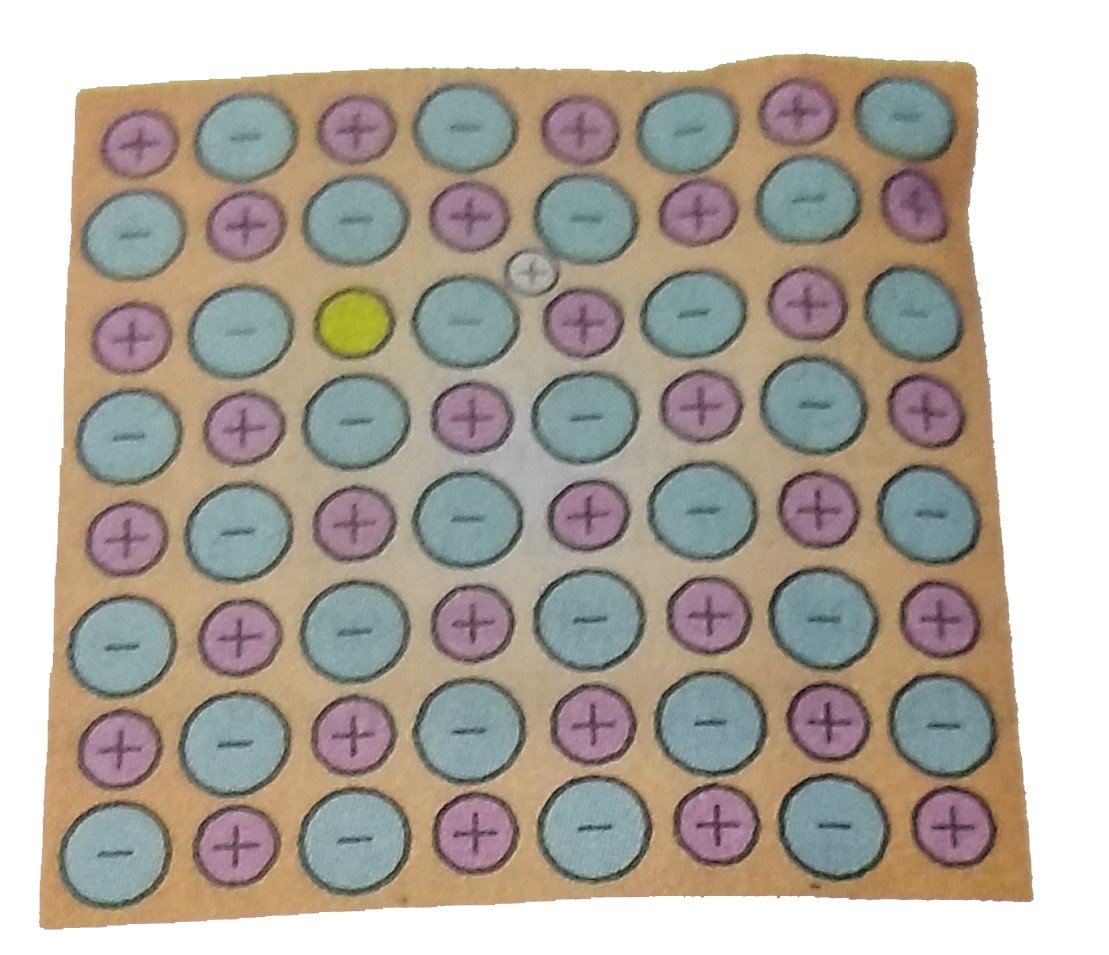
(a) Frenkel defect since cation size is smallar than that of the anion.
(b) No change in density since cation size is smaller than that of the anion.
(c) ZnS.
Similar Questions
What is the effect of Schottky defect on the density of crystal ?
View SolutionExamine the illustration of a portion of the deffective crystal and answer the following question :
(a) What are these type of vacancy defects called ?
(b) How is the density of a crystal affected by these defects ?
(c) Name one ionic compound which can show this type of defect in the crystalline state.
(d) How is the stoichiometry of the compound affected ?
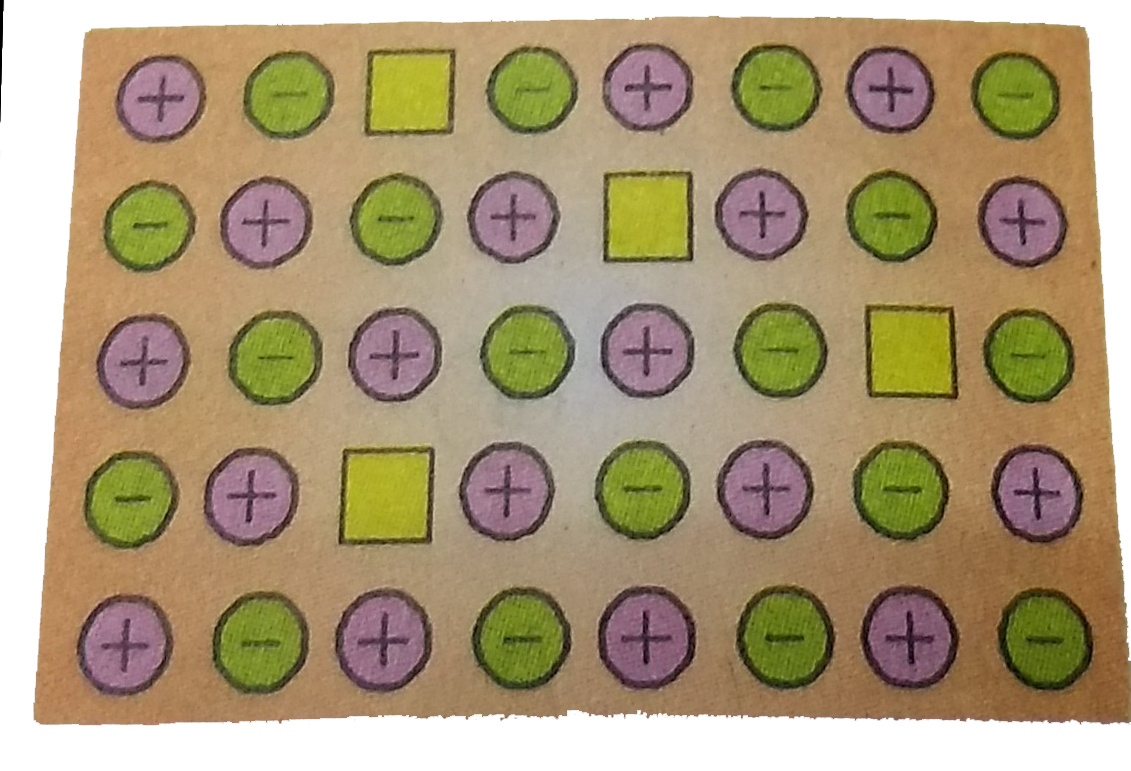
View Solution
Knowledge Check
What is the effect of Frenkel defect on the density of ionic solids?
AThe density of the crystal increasesBThe density of the crystal decreasesCThe density of the crystal remains unchangedDThere is no relationship between density of a crystal and defect present in itThe crystal showing Frenkel defect is:
A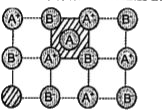 B
B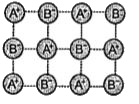 C
C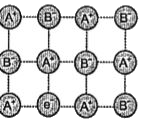 D
D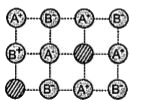
What is the non-stoichiometry defect in the crystals ?
View SolutionName the defect in the following crystal:
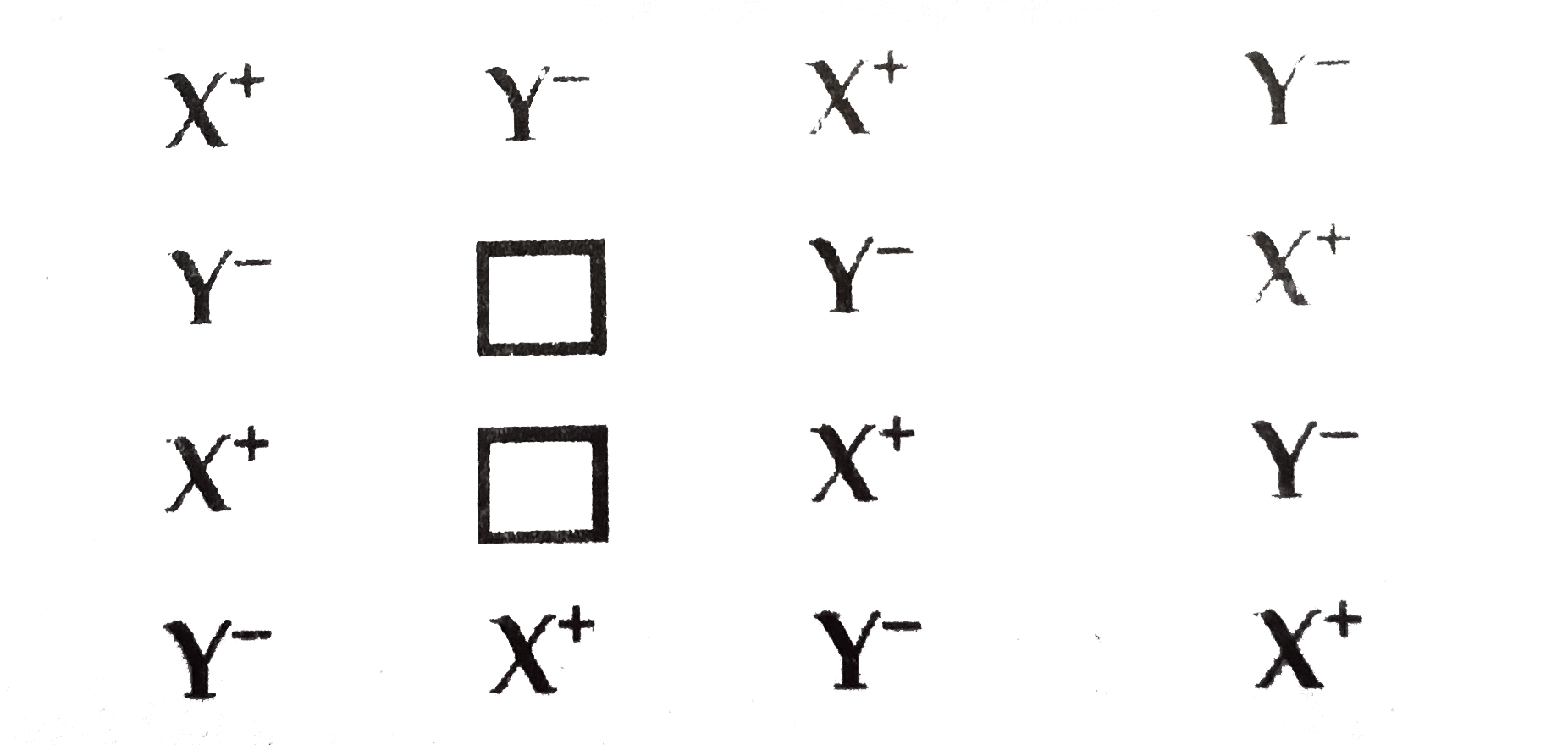
View SolutionName some other defects of eye.
View SolutionExamine the given defective crystal :

Answer the following questions :
(i) Is the above defect stoichiometric or non-stoichiometric ?
(ii) Write the term used for this type of defect. Give an example of the compound which shows this type of defect.
(iii) How does this defect affect the density of the crystal ?
View SolutionGiven below is a diagram depicting a defect of the human eye, study the same and then answer the questions that follow:
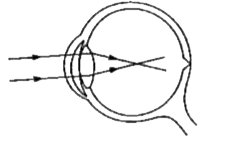
(i) Name the defect shown in the diagram.
(ii) What are the two possible reasons that cause this defect?
(iii) Name the type of lens used to correct this defect.
(iv) With the help of a diagram show how the defect shown above is rectified using a suitable lens.
View SolutionWhat is Schottky defect in a solid ?
View Solution
