Complete the following reactions
(i) CH3−CH2−CH2−CH2−CHONi/H2−−−→A
To complete the reaction given in the question, we start with the compound pentanal, which has the structure CH3−CH2−CH2−CH2−CHO.
1. Identify the Reactant: The reactant is pentanal, which is an aldehyde with the formula C5H10O. Its structure can be represented as:
CH3−CH2−CH2−CH2−CHO
2. Identify the Reaction Conditions: The reaction is carried out in the presence of hydrogen gas (H2) and a catalyst (Ni, nickel). This indicates that the reaction is a reduction process.
3. Understand the Reduction Process: Aldehydes can be reduced to primary alcohols when treated with hydrogen in the presence of a catalyst. In this case, pentanal will be reduced to pentanol.
4. Write the Product Structure: The reduction of pentanal (an aldehyde) will yield pentanol (a primary alcohol). The structure of pentanol is:
CH3−CH2−CH2−CH2−CH2−OH
5. Final Product: Therefore, the product A after the reduction of pentanal is:
CH3−CH2−CH2−CH2−CH2−OH(pentanol)
Final Answer:
The product A is pentanol, which can be represented as:
CH3−CH2−CH2−CH2−CH2−OH
CH3−CH2−CH2−CH2−CHO
CH3−CH2−CH2−CH2−CH2−OH
CH3−CH2−CH2−CH2−CH2−OH(pentanol)
The product A is pentanol, which can be represented as:
CH3−CH2−CH2−CH2−CH2−OH
Similar Questions
Knowledge Check
Complete the following reactions:
(i) C2H5I+H2Zn−Cu−−−−→C2H5OHK
(ii) CH30COONa+H2OElectrolysis−−−−−−−→Y
(iii) CH3CH2CH2CH2CH2CH3Cr2O3/Al2O3−−−−−−−−→773KZAX=C2H6,Y=C2H6,Z=C6H6BX=CH4,Y=CH30COOH,Z=CH3CH3CX=C2H6,Y=CH4,Z=C4H10DX=C2H6,Y=CH4,Z=C5H10
Complete the following reaction :
(CH3CH2COO)2Caheat−−→
View SolutionWhat will be product in the following reaction
CH3−CH2−CH2−CH3KMnO4−−−−→?
View SolutionComplete the follwing reaction equations :
(i)
(ii) CH3CH2CH=CH2+HBr→
View SolutionComplete the following reaction :
CH3CHOLiAlH4−−−→
View SolutionComplete the following reactions
(i) CH3−O∣∣C−CH3CH3MgBr−−−−−→ether(dry)AH3O+−−−→B (ii) CH3−CH2−CH2−CHONi/H2−−−→A
View SolutionComplete the following reactions:
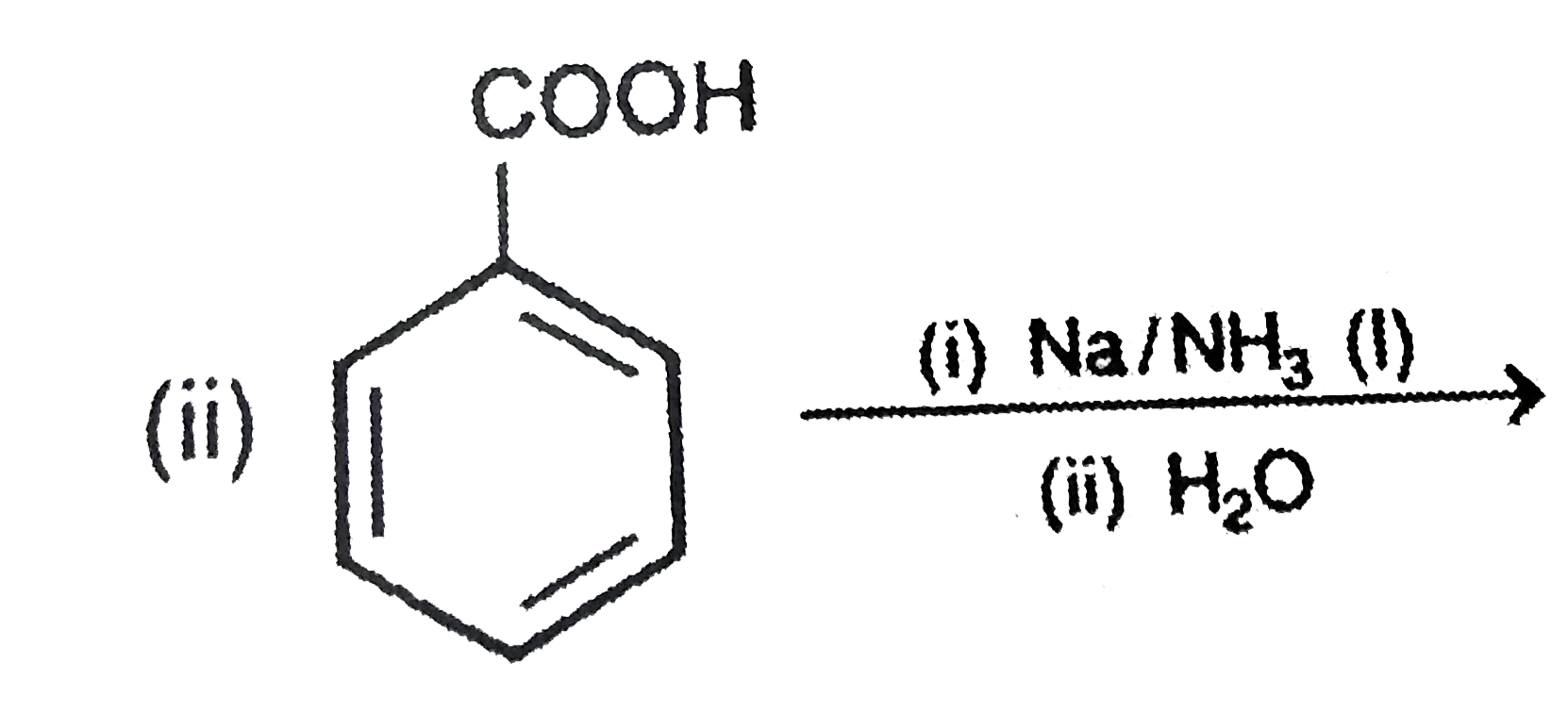
(i)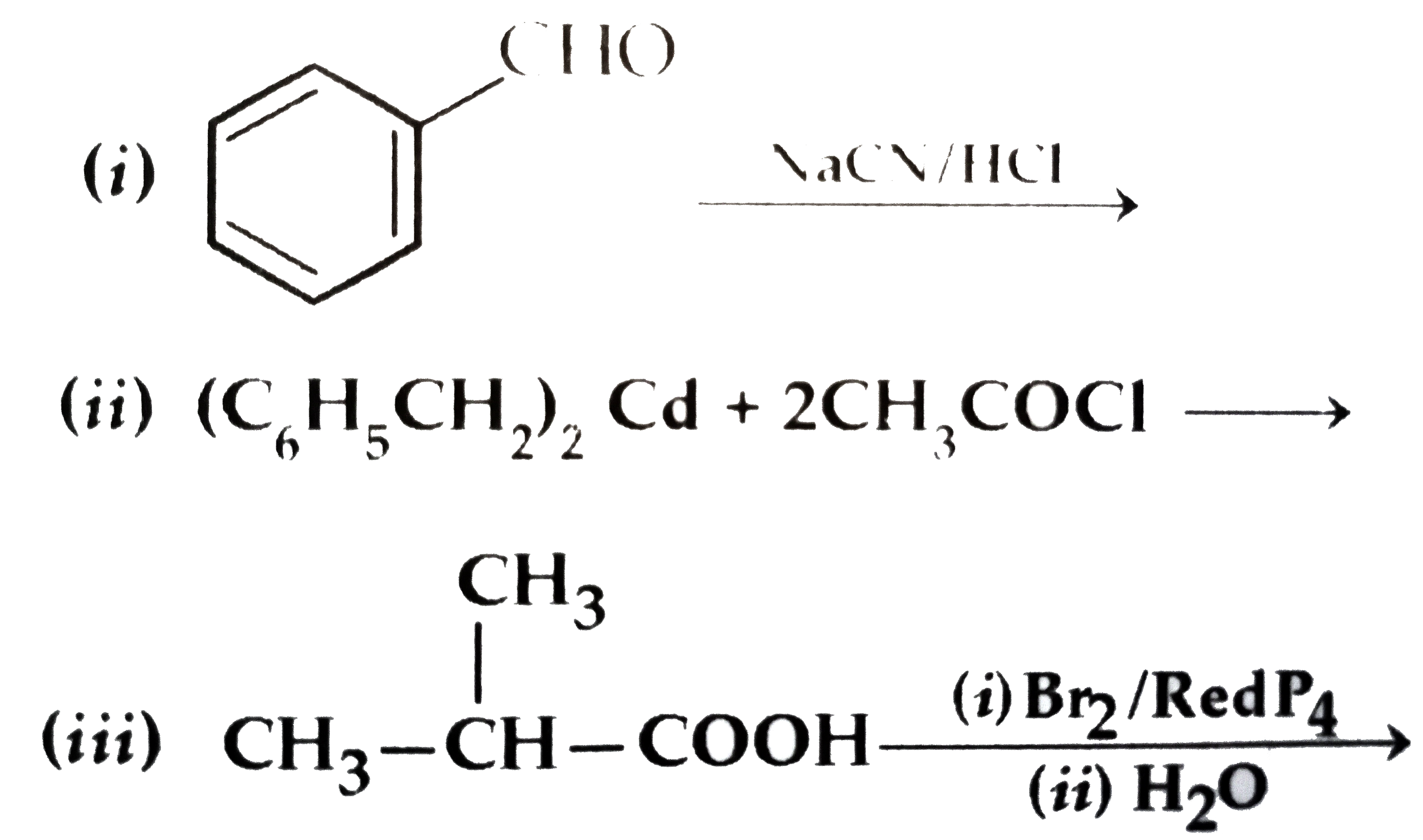
(ii) (C6H5CH2)2Cd+2CH3COC<o
(iii) CH3−CH3∣CH−COOH(i)Br2/RedP4−−−−−−−−−→(ii)H2O
View SolutionPredict the products of the following reactions:
(i) CH3−CH2−CH2−O−CH3+HBr→
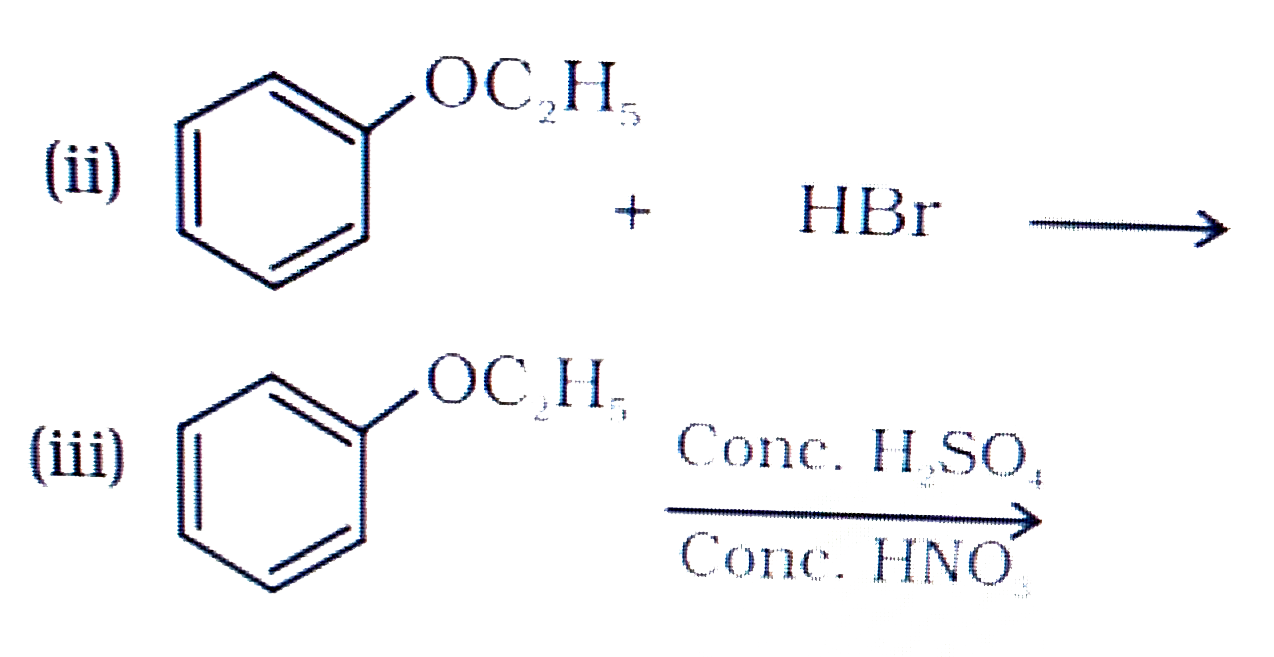
(iv) (CH3)3C−OC2H5HI−−→
View Solution