Consider a PV-diagram in which the path followed by one mole of perfect gas a cylindrical container is shown in (fig.).
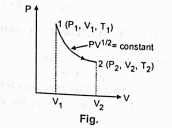 What is the ratio of temperature T1/T2 ,if V2=2V1?Solution in Punjabi
What is the ratio of temperature T1/T2 ,if V2=2V1?Solution in Punjabi
Step by step video solution for Consider a PV-diagram in which the path followed by one mole of perfect gas a cylindrical container is shown in (fig.).What is the ratio of temperature T_1 // T_2 ,if V_2 = 2V_1? by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Which state of matter has the greatest and least internal enrgy having the same mass and same temperature?
View SolutionWill you prefer a thin or thick handle to carry you bag and why ?
View SolutionWhat is SI unit of work?
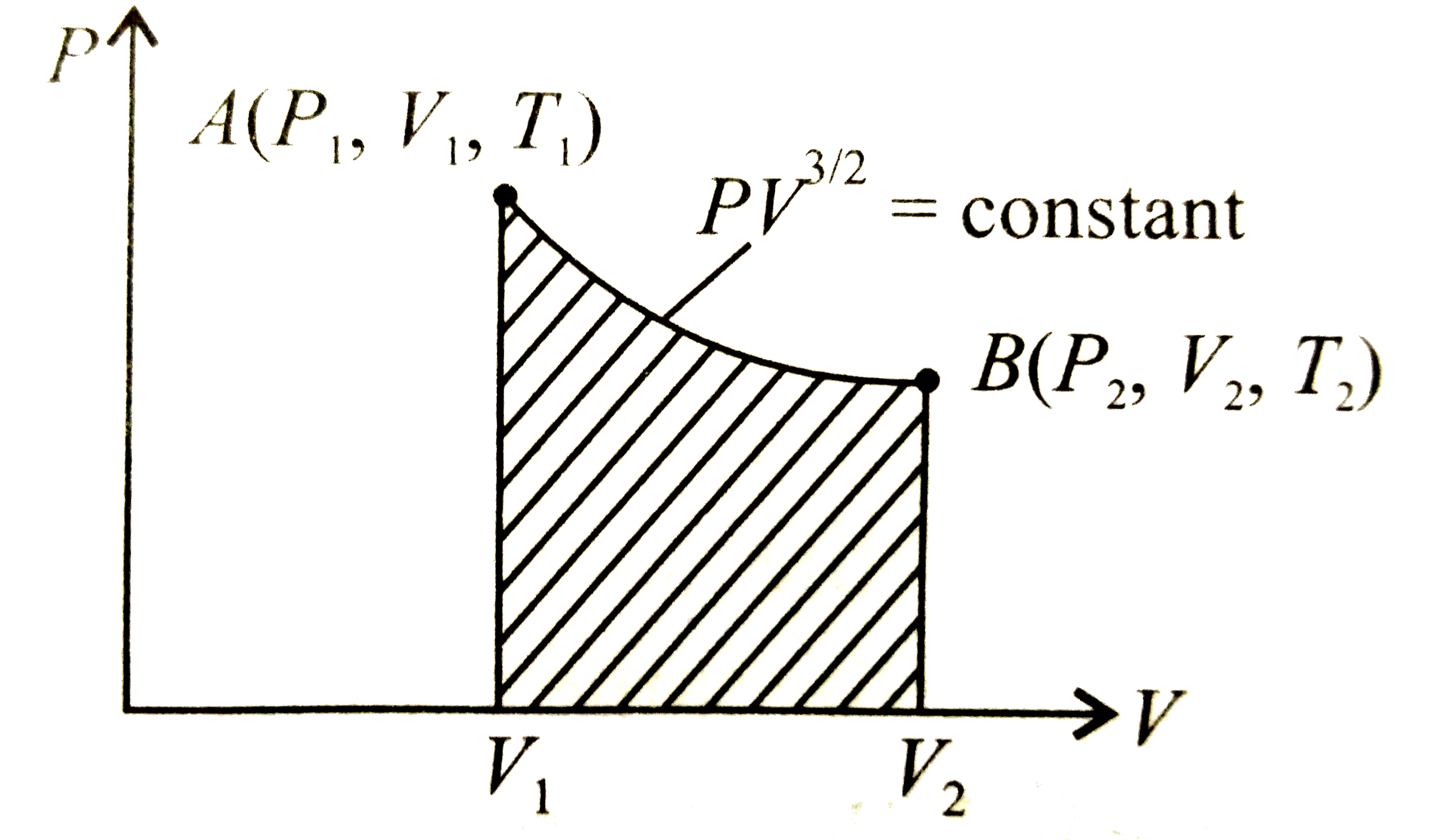
View SolutionOne mole of a gas in the state A(P_1, V_1, T_1) is subjected to adiabatic expansion to attain state B(P_2,V_2,T_2) . The work done by the gas is:
View SolutionThe P-V diagram of path followed by one mole of perfect gas in a cylindrical container is hsown in figure, the work done when the gas is taken from state A to state B is
View SolutionOne mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by :
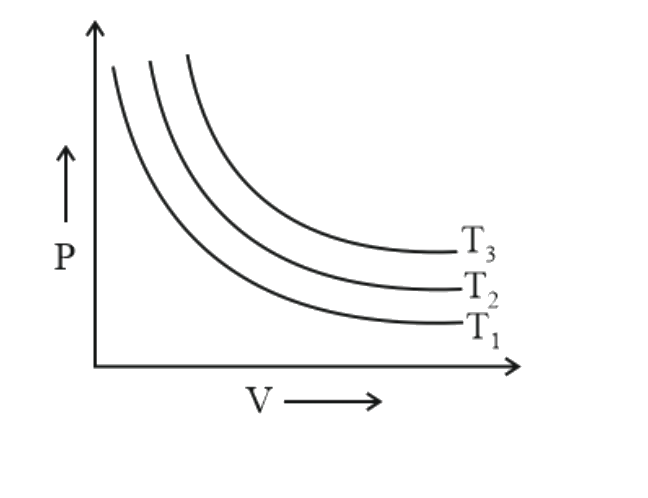
View Solutionfor the given PV isotherms , which of the following is correct for T1,T2,T3 ?
View SolutionThe product (PV) is plotted against P at two temperature T1andT2 and the result is given in following figure
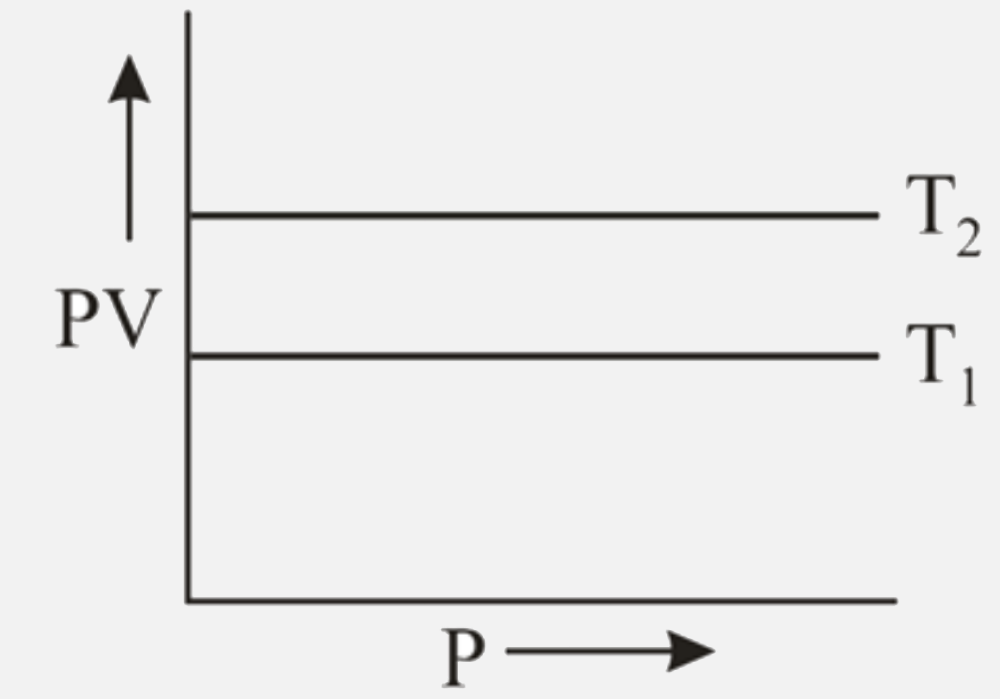
What is correct about T1andT2 ?
View SolutionTension for the body shown in Fig. T_1 And T_2 Is the ratio of
View SolutionConsider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical container is shown in figure.
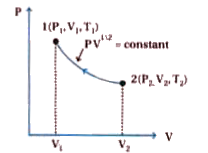
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperature T1T2 if V2=2V1 ?
(c) Given the internal energy for one mole of gas at temperature T is 32RT, find the heat 2 supplied to the gas when it is taken from state 1 to 2, with V2=2V1
View SolutionOne mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by :
View SolutionThe P-V diagram of path followed by one mole of perfect gas in a cylindrical container is shown in figure, the work done when the gas is taken from state A to state B is

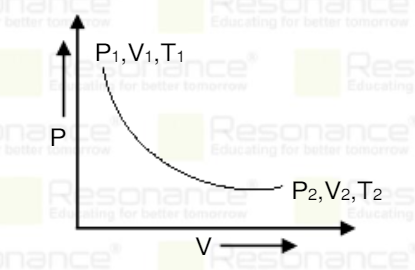
View Solutionusing the following graph for an ideal gas follow PV12 = constant . find P1/P2 ratio. If V2=2V1
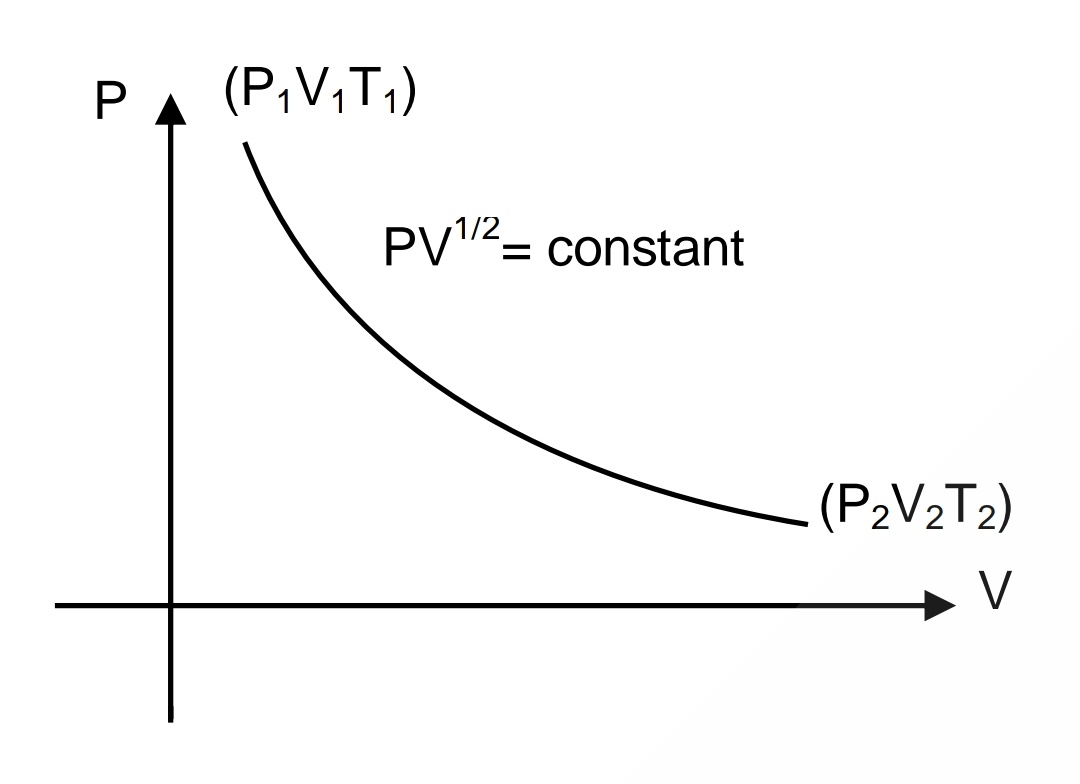
View SolutionA gas follows PV12=constant as shown. If V2=2V1 , find T2T1
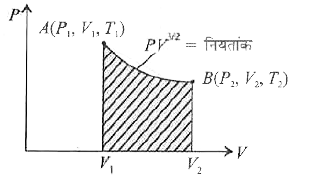
View Solutionचित्र में किसी बेलनाकार पात्र में आदर्श गैस के 1 मोल द्वारा तय किए गये मार्ग के P-V ग्राफ को दर्शाया गया है।
गैस को स्थिति A से B में ले जाने के लिए किया गया कार्य होता है-
View SolutionOne mole of an ideal gas expands adiabatically from temperature T_1 to T_2 .the workdone by the gas is_____.
View Solution

