The optical rotation of the ∝- form of a pyranose is +150.70, that of the β-form is +52.80. In solution and equilibrium mixture of the anomers has an optical rotation of +80.20. The percentage of the ∝- form at equilibrium is
0.28
0.32
0.68
0.72
The correct Answer is:A
150.7(X)+52.8(1+X)=+80.2
150.7X+52.8−52.8X=+80.2
97.9X=27.4
X=0.279=0.28
Similar Questions
The specific rotation for the α-anomer of a carbohydrate is +29∘ and that of β-anomer is −17∘. Due to mutarotation, the equilibrium specific rotation is +14∘. The percentage of α-anomer is
View SolutionSpecific rotation for alpha -anomer of a given mono saccharide is 100^(@) and for beta -anomer is +20^(@) , specific rotation of equilibrium mixture is 68^(@) , if % of alpha anomer is x xx 10% then 'x' is
View SolutionThe optical rotation of the alpha form of a pyranose is + 150.70, that of the beta form is +52.80. In solution an equlibrium mixture of the anomers has an optical rotation of +80.20. The percentage of the alpha form at equilibrium is
View Solution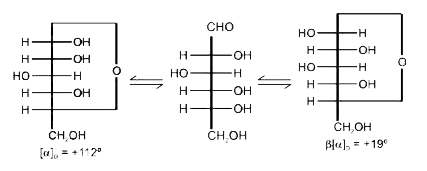
उपरोक्त प्रक्रिया जिसमें α और β रूप अचक्रिय रूप एक साथ साम्य अवस्था में रहते हैं और प्रकाशिक घूर्णन में परिवर्तन देखा जाता है। इसे कहते है -
View Solutionएक पाइरैनोस के α - रूप का प्रकाशिक घूर्णन +150.70 है,β - रूप का प्रकाशिक घूर्णन +52.80 है। विलयन में एनोमर के साम्य मिश्रण का प्रकाशिक घूर्णन +80 .20 होता है। साम्य पर α - रूप का प्रतिशत है:
View SolutionThe specific rotation of two glucose anomers are α=+110∘ and β=19∘ and for the constant equilibrium mixtures is +52.7∘. Calculate the percentage compositions of the anomers in the equilibrium mixture.
View SolutionThe above process in which alpha and beta form remain in equilibrium with acyclic form and a change in optical rotation is observed which is known as -
View SolutionThe optical rotation of the α-form of a pyranose is +150.7∘, that of the β-form is +52.8∘. In solution an equilibrium mixture of these anomers has an optical rotation of +80.2∘. The precentage of the α-form in equilibrium mixture is :
View Solution
