The name for HC≡C−C∣CH3=CH−CH3 is :-
3-Methyl-2-penten-4-yne
3-Methyl-3-penten-1-yne
3-Methyl-4-pentyn-1-ene
3-Methyl pentenyne
The correct Answer is:B
To determine the IUPAC name for the compound HC≡C−CH(CH₃)−C=CH−CH₃, we will follow the systematic approach for naming organic compounds that contain both double and triple bonds.
1. Identify the Longest Carbon Chain:
- The longest continuous carbon chain that includes both the triple bond (alkyne) and the double bond (alkene) consists of 5 carbon atoms.
2. Number the Carbon Chain:
- Start numbering the carbon chain from the end closest to the triple bond to give it the lowest possible number.
- The numbering will be as follows:
- C1: HC≡C (triple bond)
- C2: CH
- C3: C(CH₃) (methyl group attached)
- C4: C=CH (double bond)
- C5: CH₃
3. Identify the Functional Groups:
- The compound has a triple bond (alkyne) and a double bond (alkene).
- The triple bond takes precedence over the double bond in naming.
4. Assign Locants to the Functional Groups:
- The triple bond is at position 1 (C1).
- The double bond is at position 4 (C4).
- The methyl group is at position 3 (C3).
5. Construct the Name:
- According to IUPAC nomenclature, we list the substituents in alphabetical order, followed by the parent chain name.
- The name will be constructed as follows:
- The substituent is "3-methyl".
- The parent chain is "pent" (for 5 carbons).
- The suffix for the double bond is "ene" and for the triple bond is "yne".
- Since the triple bond takes precedence, we will name it as "1-yne" and "4-ene".
6. Final Name:
- Combine all parts: "3-methyl-4-pentene-1-yne".
Thus, the IUPAC name for the compound is 3-methyl-4-pentene-1-yne.
- The longest continuous carbon chain that includes both the triple bond (alkyne) and the double bond (alkene) consists of 5 carbon atoms.
- Start numbering the carbon chain from the end closest to the triple bond to give it the lowest possible number.
- The numbering will be as follows:
- C1: HC≡C (triple bond)
- C2: CH
- C3: C(CH₃) (methyl group attached)
- C4: C=CH (double bond)
- C5: CH₃
- The compound has a triple bond (alkyne) and a double bond (alkene).
- The triple bond takes precedence over the double bond in naming.
- The triple bond is at position 1 (C1).
- The double bond is at position 4 (C4).
- The methyl group is at position 3 (C3).
- According to IUPAC nomenclature, we list the substituents in alphabetical order, followed by the parent chain name.
- The name will be constructed as follows:
- The substituent is "3-methyl".
- The parent chain is "pent" (for 5 carbons).
- The suffix for the double bond is "ene" and for the triple bond is "yne".
- Since the triple bond takes precedence, we will name it as "1-yne" and "4-ene".
- Combine all parts: "3-methyl-4-pentene-1-yne".
Topper's Solved these Questions
- IUPAC NOMENCLATUREBook:ALLENChapter:IUPAC NOMENCLATUREExercise:Exercise - 03
Explore 3 Videos
- IUPAC NOMENCLATUREBook:ALLENChapter:IUPAC NOMENCLATUREExercise:Exercise - 04
Explore 4 Videos
- IUPAC NOMENCLATUREBook:ALLENChapter:IUPAC NOMENCLATUREExercise:Exercise - 01
Explore 20 Videos
- ATOMIC STRUCTUREBook:ALLENChapter:ATOMIC STRUCTUREExercise:Exercise - 05[A]
Explore 14 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:Exercise - 05(B)
Explore 8 Videos
Similar Questions
Knowledge Check
Correct name for the given compound
CH3−CH2−C∣CH2CH3H−CH2−C∣CH3H−CH2−CH3 isA3-ethyl-5-methylheptaneB5-ethyl-3-methylheptaneC1,1-diethyl-3-methylpentaneD3-methyl-5,5-diethylpentaneThe IUPAC name of CH3−C∣CH3H−CH2−O∣∣C−Br is
A2-Methylbutanoyl bromideB2-Methylbutan-4-oyl bromideC1-Bromo-3-MethylbutanoneD3-Methylbutanoyl bromide
IUPAC name of CH3−CH∣CH3−CH2−CH∣CH3−CH3 is
View SolutionWrite its IUPAC name
CH3−CH∣CH3−CH2−CH3∣C∣CH2−CH3
View SolutionName the following compounds according to IUPAC system.
(i) CH3−CH2−C | CH2ClH−CH2OH| C H−C| CH3H−CH3 (ii) CH3−C | CH3H−CH2−C | OHH−CH2OH| C H−CH3
(iii)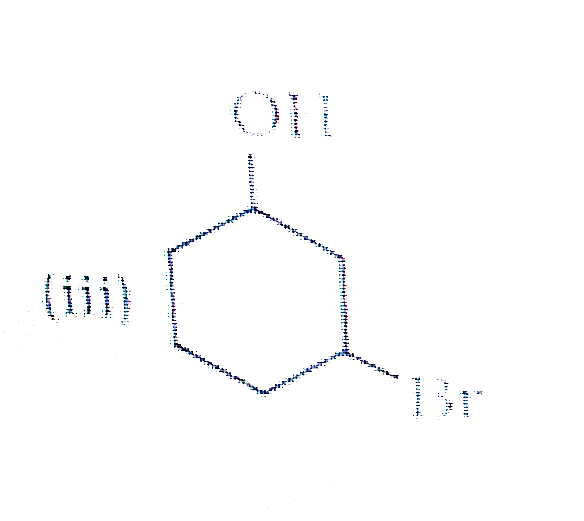
(iv) H2C=CH−C | OHH−CH2−CH2−CH3 (v) CH3−C | CH3=C | Br−CH2OH
View SolutionIUPAC name of CH3−C∣(CH2CH3)H−CH2−C∣CNH−CH3 is
View SolutionGive IUPAC names of the following compounds:
(i) CH3−C∣ClH−C∣ CH3H−C∣ CH3H−CH2OH
(ii) CH3−C∣ CH3H−O−CH2CH3

View SolutionIUPAC name of CH3−C∣Cl=CH−CH2−C∣∣O−CH3 is
View Solution

_S01_015_Q01.png)