In the compound HC≡C−CH2−CH=CH−CH3, the C2−C3 bond is the type of :-
sp−sp2
sp3−sp3
sp−sp3
sp2−sp2
The correct Answer is:C
To determine the type of bond between C2 and C3 in the compound HC≡C−CH₂−CH=CH−CH₃, we will analyze the structure and hybridization of the involved carbon atoms.
Step 1: Identify the structure of the compound.
- The compound can be represented as follows:
H−C≡C−CH₂−CH=CH−CH₃
- Here, we can see that there are both a triple bond (between C1 and C2) and a double bond (between C4 and C5).
Step 2: Number the carbon atoms.
- We will number the carbon atoms starting from the end closest to the first double or triple bond:
- C1: H
- C2: C (triple bond)
- C3: C (single bond, CH₂)
- C4: C (double bond)
- C5: C
- C6: C (CH₃)
Step 3: Determine the type of bond between C2 and C3.
- The bond between C2 and C3 is a single bond because:
- C2 is involved in a triple bond with C1 (C≡C), which consists of one sigma bond and two pi bonds.
- C3 is bonded to C2 with a single bond (C-C), which is a sigma bond.
Step 4: Identify the hybridization of C2 and C3.
- For C2:
- C2 is part of the triple bond (C≡C), which has sp hybridization. This means it has two pi bonds and one sigma bond.
- For C3:
- C3 is bonded to C2 with a single bond and has two other hydrogen atoms attached (CH₂), indicating sp³ hybridization. This means it has four sigma bonds.
Step 5: Conclude the type of bond between C2 and C3.
- The bond between C2 and C3 is a sigma bond (C-C), as it is a single bond.
Final Answer:
The bond between C2 and C3 is a single bond (sigma bond).
- The compound can be represented as follows:
H−C≡C−CH₂−CH=CH−CH₃
- Here, we can see that there are both a triple bond (between C1 and C2) and a double bond (between C4 and C5).
- We will number the carbon atoms starting from the end closest to the first double or triple bond:
- C1: H
- C2: C (triple bond)
- C3: C (single bond, CH₂)
- C4: C (double bond)
- C5: C
- C6: C (CH₃)
- The bond between C2 and C3 is a single bond because:
- C2 is involved in a triple bond with C1 (C≡C), which consists of one sigma bond and two pi bonds.
- C3 is bonded to C2 with a single bond (C-C), which is a sigma bond.
- For C2:
- C2 is part of the triple bond (C≡C), which has sp hybridization. This means it has two pi bonds and one sigma bond.
- For C3:
- C3 is bonded to C2 with a single bond and has two other hydrogen atoms attached (CH₂), indicating sp³ hybridization. This means it has four sigma bonds.
- The bond between C2 and C3 is a sigma bond (C-C), as it is a single bond.
The bond between C2 and C3 is a single bond (sigma bond).
Topper's Solved these Questions
- IUPAC NOMENCLATUREBOOK - ALLENCHAPTER - IUPAC NOMENCLATUREEXERCISE - Exercise - 03
3 Videos
- IUPAC NOMENCLATUREBOOK - ALLENCHAPTER - IUPAC NOMENCLATUREEXERCISE - Exercise - 04
4 Videos
- IUPAC NOMENCLATUREBOOK - ALLENCHAPTER - IUPAC NOMENCLATUREEXERCISE - Exercise - 01
20 Videos
- ATOMIC STRUCTUREBOOK - ALLENCHAPTER - ATOMIC STRUCTUREEXERCISE - Exercise - 05[A]
14 Videos
- MOLE CONCEPTBOOK - ALLENCHAPTER - MOLE CONCEPTEXERCISE - Exercise - 05(B)
8 Videos
Similar Questions
In the compound
CH2=CH−CH2−CH2−C≡CH the C2−C3 bond is of
View SolutionGive the IUPAC name of the compound
CH3−C≡C−CH2−CH2−CH3
View SolutionIn the organic compound CH2=CH–CH2–CH2–C≡CH, the pair of hydridised orbitals involved in the formation of: C2–C3 bond is:
(a)sp–sp2(b)sp–sp3(c)sp2–sp3(d)sp3–sp3
View SolutionWhat is the kind of isomerism exhibited by the compounds given below?
CH3−C≡C−CH2−CH2−CH3,<br>CH3−CH2−C≡C−CH2−CH3
View SolutionWrite IUPAC names of the following compounds : (a) CH3CH=C(CH3)2
(b) CH2=CH−C≡C−CH3
(c )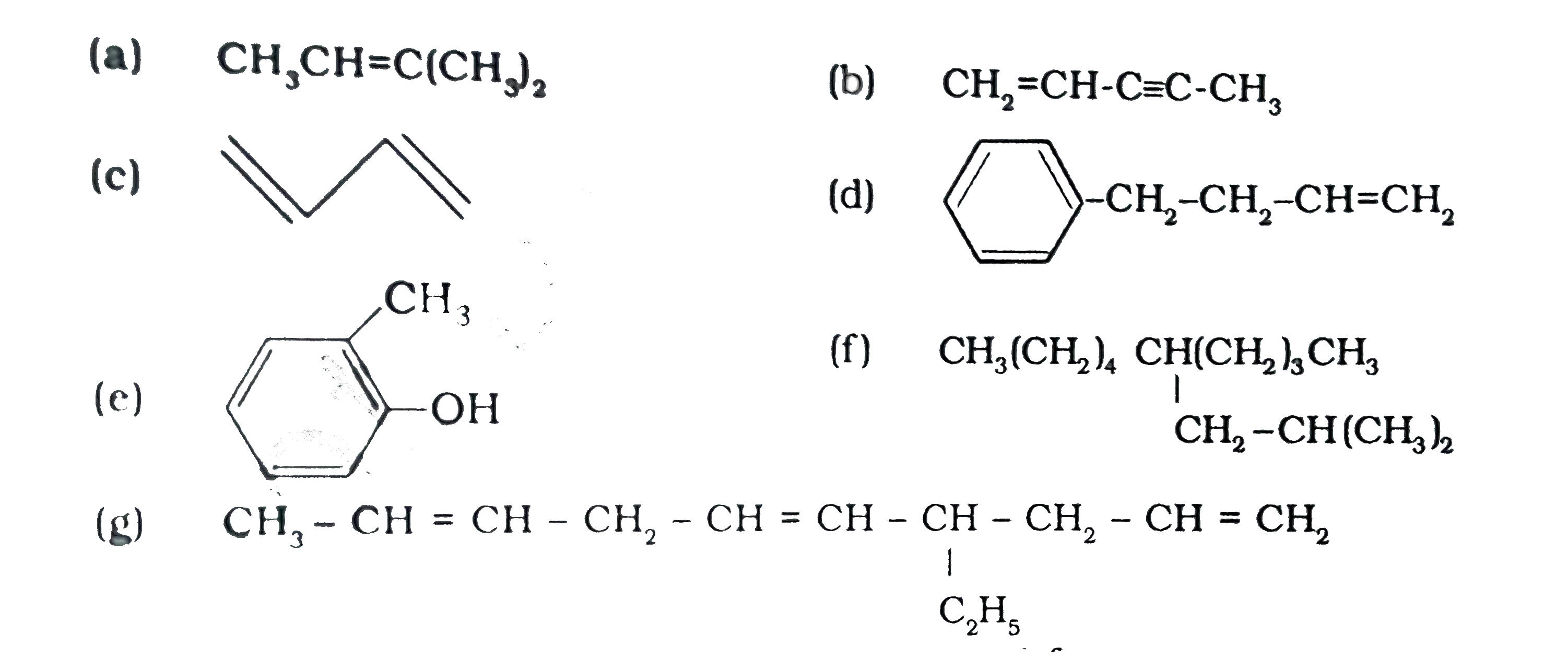
(d)
(e )
CH3(CH2)4CH(CH2)3CH3 ∣ CH2−CH(CH3)2
View SolutionThe IUPAC name of compound CH3−C(CH3)2−CH2−CH=CH2 is
View SolutionGive the IUPAC name of the compound
CH3−CH2−C(CH3)2−CH2−CH3
View SolutionGive the IUPAC name of the compound
CH3−CH2−C≡C−CH2−CH2−CH2−CH3
View SolutionIn the given compounds
CH3CH2CH2CH2Br (a)
CH3CH2CHBr−CH3 (b)
(CH3)3C−Br(c)
The correct order of boiling point is
(a)>(b)>(c)
(b)>(a)>(c)
(c)>(a)>(b)
(a)>(c)>(b)
View SolutionThe IUPAC name name of compound
CH3−CH=C−CH2−CH3 | CH2−CH2−CH3
View Solution

