Which of the following pair is having planar structure
SF4,XeF4
H3O+,SO2
BF3,XeOF2
XeF4,NO−3
The correct Answer is:D
To determine which of the given pairs has a planar structure, we can analyze the molecular geometry of each option based on their valence electrons and hybridization.
1. Analyze Option 1: SF4 and XeF4
- SF4 (Sulfur Tetrafluoride):
- Sulfur has 6 valence electrons, and each fluorine has 7 (4 fluorines contribute 28 electrons).
- Total = 6 + 28 = 34 electrons.
- Dividing by 2 gives us 17 pairs. This means there are 4 bond pairs and 1 lone pair (34 - 32 = 2 electrons, which corresponds to 1 lone pair).
- Geometry: The arrangement is trigonal bipyramidal with one lone pair, leading to a seesaw shape, which is not planar.
- XeF4 (Xenon Tetrafluoride):
- Xenon has 8 valence electrons and each fluorine has 7 (4 fluorines contribute 28 electrons).
- Total = 8 + 28 = 36 electrons.
- Dividing by 2 gives us 18 pairs. This means there are 4 bond pairs and 2 lone pairs (36 - 32 = 4 electrons, which corresponds to 2 lone pairs).
- Geometry: The arrangement is octahedral, and with 2 lone pairs, the shape is square planar, which is planar.
Conclusion for Option 1: Not both are planar (only XeF4 is planar).
2. Analyze Option 2: H3O+ and SO2
- H3O+ (Hydronium Ion):
- Oxygen has 6 valence electrons, and each hydrogen has 1 (3 hydrogens contribute 3 electrons).
- Total = 6 + 3 - 1 (due to the positive charge) = 8 electrons.
- Dividing by 2 gives us 4 pairs. This means there are 3 bond pairs and 1 lone pair.
- Geometry: The arrangement is tetrahedral with one lone pair, leading to a pyramidal shape, which is not planar.
- SO2 (Sulfur Dioxide):
- Sulfur has 6 valence electrons, and each oxygen has 6 (2 oxygens contribute 12 electrons).
- Total = 6 + 12 = 18 electrons.
- Dividing by 2 gives us 9 pairs. This means there are 2 bond pairs and 1 lone pair.
- Geometry: The arrangement is bent due to the lone pair, which is also not planar.
Conclusion for Option 2: Not both are planar.
3. Analyze Option 3: BF3 and XeOF2
- BF3 (Boron Trifluoride):
- Boron has 3 valence electrons, and each fluorine has 7 (3 fluorines contribute 21 electrons).
- Total = 3 + 21 = 24 electrons.
- Dividing by 2 gives us 12 pairs. This means there are 3 bond pairs and no lone pairs.
- Geometry: The arrangement is trigonal planar, which is planar.
- XeOF2 (Xenon Oxyfluoride):
- Xenon has 8 valence electrons, oxygen has 6, and each fluorine has 7 (2 fluorines contribute 14 electrons).
- Total = 8 + 6 + 14 = 28 electrons.
- Dividing by 2 gives us 14 pairs. This means there are 2 bond pairs and 2 lone pairs.
- Geometry: The arrangement is trigonal bipyramidal, but with 2 lone pairs, it forms a T-shaped structure, which is planar.
Conclusion for Option 3: Both are planar.
4. Analyze Option 4: XeF4 and Nitrate Ion (NO3-)
- XeF4 (Xenon Tetrafluoride): As analyzed earlier, it is square planar.
- Nitrate Ion (NO3-):
- Nitrogen has 5 valence electrons, and each oxygen has 6 (3 oxygens contribute 18 electrons).
- Total = 5 + 18 + 1 (due to the negative charge) = 24 electrons.
- Dividing by 2 gives us 12 pairs. This means there are 3 bond pairs and no lone pairs.
- Geometry: The arrangement is trigonal planar, which is planar.
Conclusion for Option 4: Both are planar.
Final Answer:
The pair that has a planar structure is Option 4: XeF4 and Nitrate Ion (NO3-).
- SF4 (Sulfur Tetrafluoride):
- Sulfur has 6 valence electrons, and each fluorine has 7 (4 fluorines contribute 28 electrons).
- Total = 6 + 28 = 34 electrons.
- Dividing by 2 gives us 17 pairs. This means there are 4 bond pairs and 1 lone pair (34 - 32 = 2 electrons, which corresponds to 1 lone pair).
- Geometry: The arrangement is trigonal bipyramidal with one lone pair, leading to a seesaw shape, which is not planar.
- XeF4 (Xenon Tetrafluoride):
- Xenon has 8 valence electrons and each fluorine has 7 (4 fluorines contribute 28 electrons).
- Total = 8 + 28 = 36 electrons.
- Dividing by 2 gives us 18 pairs. This means there are 4 bond pairs and 2 lone pairs (36 - 32 = 4 electrons, which corresponds to 2 lone pairs).
- Geometry: The arrangement is octahedral, and with 2 lone pairs, the shape is square planar, which is planar.
- H3O+ (Hydronium Ion):
- Oxygen has 6 valence electrons, and each hydrogen has 1 (3 hydrogens contribute 3 electrons).
- Total = 6 + 3 - 1 (due to the positive charge) = 8 electrons.
- Dividing by 2 gives us 4 pairs. This means there are 3 bond pairs and 1 lone pair.
- Geometry: The arrangement is tetrahedral with one lone pair, leading to a pyramidal shape, which is not planar.
- SO2 (Sulfur Dioxide):
- Sulfur has 6 valence electrons, and each oxygen has 6 (2 oxygens contribute 12 electrons).
- Total = 6 + 12 = 18 electrons.
- Dividing by 2 gives us 9 pairs. This means there are 2 bond pairs and 1 lone pair.
- Geometry: The arrangement is bent due to the lone pair, which is also not planar.
- BF3 (Boron Trifluoride):
- Boron has 3 valence electrons, and each fluorine has 7 (3 fluorines contribute 21 electrons).
- Total = 3 + 21 = 24 electrons.
- Dividing by 2 gives us 12 pairs. This means there are 3 bond pairs and no lone pairs.
- Geometry: The arrangement is trigonal planar, which is planar.
- XeOF2 (Xenon Oxyfluoride):
- Xenon has 8 valence electrons, oxygen has 6, and each fluorine has 7 (2 fluorines contribute 14 electrons).
- Total = 8 + 6 + 14 = 28 electrons.
- Dividing by 2 gives us 14 pairs. This means there are 2 bond pairs and 2 lone pairs.
- Geometry: The arrangement is trigonal bipyramidal, but with 2 lone pairs, it forms a T-shaped structure, which is planar.
- XeF4 (Xenon Tetrafluoride): As analyzed earlier, it is square planar.
- Nitrate Ion (NO3-):
- Nitrogen has 5 valence electrons, and each oxygen has 6 (3 oxygens contribute 18 electrons).
- Total = 5 + 18 + 1 (due to the negative charge) = 24 electrons.
- Dividing by 2 gives us 12 pairs. This means there are 3 bond pairs and no lone pairs.
- Geometry: The arrangement is trigonal planar, which is planar.
The pair that has a planar structure is Option 4: XeF4 and Nitrate Ion (NO3-).
টপার এই প্রশ্নগুলো সমাধান করেছে
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 472 Videos
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 472 Videos
- Chemical EquilibriumBook:ALLENChapter:Chemical EquilibriumExercise:All Questions
Explore 30 Videos
- ELECTROCHEMISTRYBook:ALLENChapter:ELECTROCHEMISTRYExercise:EXERCISE -05 [B]
Explore 38 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Which of the following is planar?
ASiCl4BNF3CSF4DXeF4Which one of the following pairs of plant structures has haploid number of chromosomes?
AMegaspore mother cells and antipodal cellsBEgg cell and antipodal cellsCNucellus and antipodal cellsDEgg nucleus and secondary nucleusWhich of the following is pair of resonance structure?
A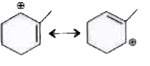 B
B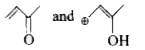 C
C D
D
Which of the following is not planar in structure?
View SolutionWhich of the following has square planar structure
View SolutionWhich of the following organ is a paired structure ?
View SolutionWhich of the following set of species have planar structure?
View SolutionWhich of the following is a planar molecule ?
View Solution
