Which reaction is not used in the preparation of H2?
Zn+NaOH→
Mg+NaOH→
Al+NaOH→
Be+NaOH→
The correct Answer is:B
To determine which reaction is not used in the preparation of hydrogen gas (H₂), we will analyze the reactions of various metals with sodium hydroxide (NaOH) and see if they produce hydrogen gas.
1. Identify the Metals and Reactions: We will consider the reactions of the following metals with sodium hydroxide:
- Zinc (Zn)
- Magnesium (Mg)
- Aluminium (Al)
- Beryllium (Be)
2. Reaction of Zinc with NaOH:
- The reaction is:
Zn+2NaOH+2H2O→Na2Zn(OH)4+H2
- This reaction produces hydrogen gas (H₂). Therefore, zinc is a valid method for hydrogen preparation.
3. Reaction of Magnesium with NaOH:
- The reaction is:
Mg+2NaOH+2H2O→Na2Mg(OH)4+H2
- However, magnesium does not react with NaOH to produce hydrogen gas. Thus, this reaction does not yield H₂.
4. Reaction of Aluminium with NaOH:
- The reaction is:
2Al+2NaOH+6H2O→2Na3Al(OH)6+3H2
- This reaction produces hydrogen gas (H₂). Therefore, aluminium is also a valid method for hydrogen preparation.
5. Reaction of Beryllium with NaOH:
- The reaction is:
Be+2NaOH→Na2BeO2+H2
- This reaction also produces hydrogen gas (H₂). Thus, beryllium is a valid method for hydrogen preparation.
6. Conclusion:
- Among the metals considered, magnesium does not produce hydrogen gas when reacted with sodium hydroxide. Therefore, the reaction of magnesium with NaOH is not used in the preparation of H₂.
Final Answer:
The reaction that is not used in the preparation of H₂ is the reaction of Magnesium with NaOH.
---
- Zinc (Zn)
- Magnesium (Mg)
- Aluminium (Al)
- Beryllium (Be)
- The reaction is:
Zn+2NaOH+2H2O→Na2Zn(OH)4+H2
- This reaction produces hydrogen gas (H₂). Therefore, zinc is a valid method for hydrogen preparation.
- The reaction is:
Mg+2NaOH+2H2O→Na2Mg(OH)4+H2
- However, magnesium does not react with NaOH to produce hydrogen gas. Thus, this reaction does not yield H₂.
- The reaction is:
2Al+2NaOH+6H2O→2Na3Al(OH)6+3H2
- This reaction produces hydrogen gas (H₂). Therefore, aluminium is also a valid method for hydrogen preparation.
- The reaction is:
Be+2NaOH→Na2BeO2+H2
- This reaction also produces hydrogen gas (H₂). Thus, beryllium is a valid method for hydrogen preparation.
- Among the metals considered, magnesium does not produce hydrogen gas when reacted with sodium hydroxide. Therefore, the reaction of magnesium with NaOH is not used in the preparation of H₂.
The reaction that is not used in the preparation of H₂ is the reaction of Magnesium with NaOH.
Topper's Solved these Questions
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 472 Videos
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 472 Videos
- Chemical EquilibriumBook:ALLENChapter:Chemical EquilibriumExercise:All Questions
Explore 30 Videos
- ELECTROCHEMISTRYBook:ALLENChapter:ELECTROCHEMISTRYExercise:EXERCISE -05 [B]
Explore 38 Videos
Similar Questions
The correct IUPAC name of the following compound is :
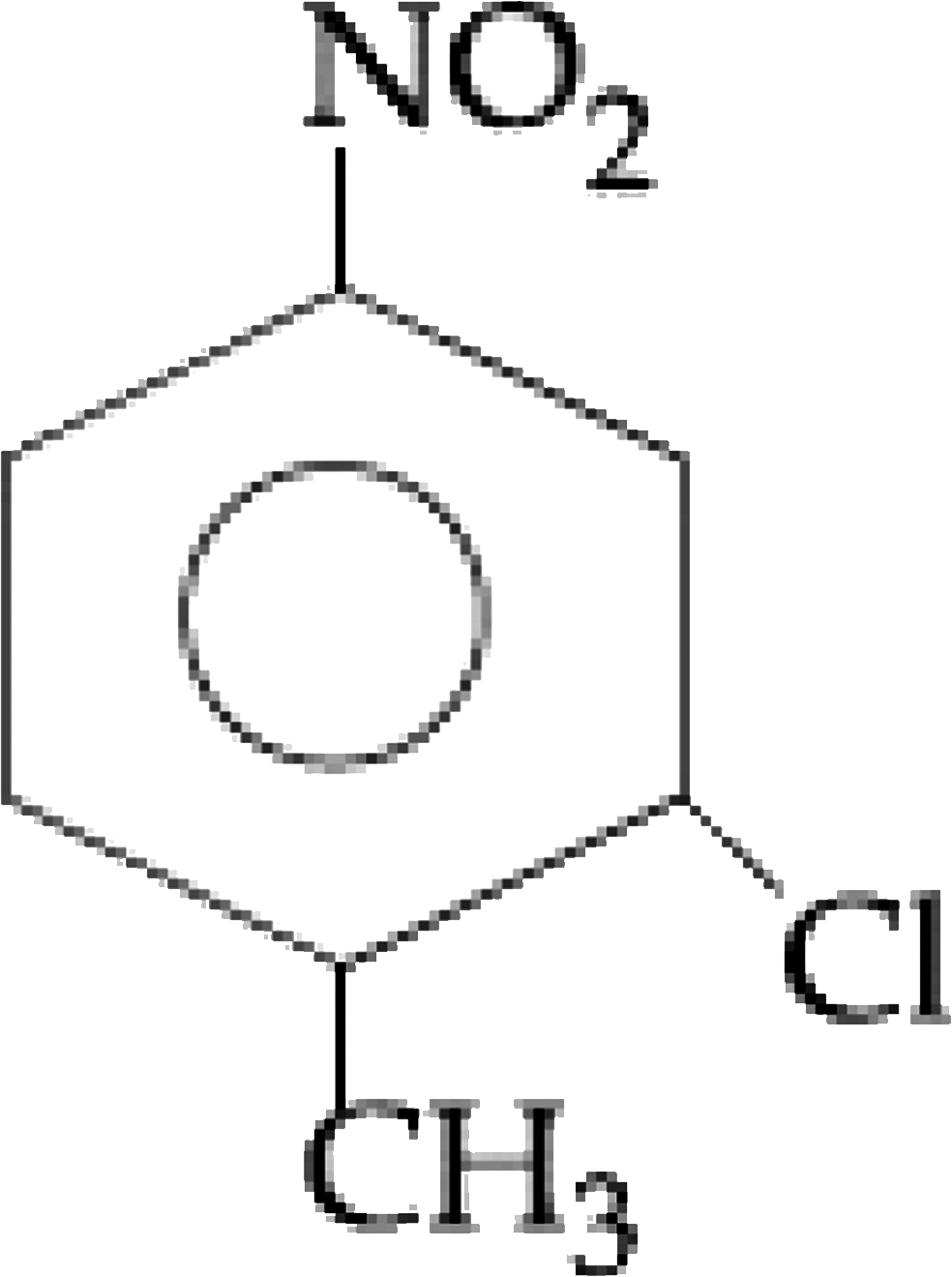
View SolutionThe correct IUPAC name of the following compound is :

View Solution48 g of SO3 , 12.8 g of SO2 and 9.6 g of O2 are present in one litre . The respective active masses will be
View SolutionFind the solubility product of a saturated solution of Ag2CrO4 in water at 298K, if the EMF of the cell :
Ag∣∣Ag⊕(satAg2CrO4sol)∣∣|Ag(0.1M)|Agis0.164V at 298K.
View Solutionwhat happens if conc H_(2)SO_(4) is used in preparing hydrogen by its reaction with a metal?
View SolutionWhich of the following reactions is used in the preparation of N2(g)?
View SolutionWhich of the following reactions is used in the preparation of N2(g)?
View SolutionWhich of following cannot be used for the preparation of H2 ?
View SolutionWhich of the following reactions is not used for the preparation of a colloidal solutions ?
View SolutionWhich catalyst is used in the preparation of CH3OH by the reaction of CO(g)&H2(g)?
View SolutionIn the preparation of sulphuric acid, V_(2)O_(5) is used in the reaction, which is
View SolutionH2O2 is commonly prepared in lab by the reaction of
View SolutionWhich of the following reactions can be used to prepare methane?
View SolutionWhich reaction is used to prepare Toluene from chlorobenzene ?
View SolutionThe substance which can not be used for preparing H2O2 solution is
View SolutionIn the preparation of H_(2) SO_(4), by Constact process V _(2) O_(5) is used as a catalyst in the reaction.
View Solution
