Which cannot be oxidised by H2O2 ?
Na2SO3
MnSO4
FeSO4
o3
The correct Answer is:D
To determine which substance cannot be oxidized by hydrogen peroxide (H₂O₂), we need to analyze the oxidation states of the elements in the given options and understand the oxidizing properties of H₂O₂.
1. Understanding Hydrogen Peroxide as an Oxidizing Agent:
- Hydrogen peroxide (H₂O₂) is known to be a strong oxidizing agent. It can decompose to produce nascent oxygen, which is capable of oxidizing various substances.
2. Analyzing the Options:
- We need to evaluate each option to see if it can be oxidized by H₂O₂.
3. Option A: SO₃²⁻ (Sulfite Ion):
- The oxidation state of sulfur in SO₃²⁻ is +4.
- It can be oxidized to SO₄²⁻ (sulfate ion), where the oxidation state of sulfur is +6.
- Therefore, H₂O₂ can oxidize SO₃²⁻.
4. Option B: MnSO₄ (Manganese(II) Sulfate):
- The oxidation state of manganese (Mn) in MnSO₄ is +2.
- It can be oxidized to MnO₄⁻ (permanganate ion), where the oxidation state of Mn is +7.
- Hence, H₂O₂ can oxidize MnSO₄.
5. Option C: Ferrous Ion (Fe²⁺):
- The oxidation state of iron (Fe) in ferrous ion is +2.
- It can be oxidized to ferric ion (Fe³⁺), where the oxidation state of Fe is +3.
- Thus, H₂O₂ can oxidize Fe²⁺.
6. Option D: Ozone (O₃):
- Ozone is a powerful oxidizing agent itself.
- Since H₂O₂ is less powerful than ozone, it cannot oxidize ozone. Instead, ozone can act as an oxidizing agent and may reduce H₂O₂.
- Therefore, H₂O₂ cannot oxidize O₃.
7. Conclusion:
- The substance that cannot be oxidized by H₂O₂ is Option D: Ozone (O₃).
Final Answer:
Option D: Ozone (O₃) cannot be oxidized by H₂O₂.
- Hydrogen peroxide (H₂O₂) is known to be a strong oxidizing agent. It can decompose to produce nascent oxygen, which is capable of oxidizing various substances.
- We need to evaluate each option to see if it can be oxidized by H₂O₂.
- The oxidation state of sulfur in SO₃²⁻ is +4.
- It can be oxidized to SO₄²⁻ (sulfate ion), where the oxidation state of sulfur is +6.
- Therefore, H₂O₂ can oxidize SO₃²⁻.
- The oxidation state of manganese (Mn) in MnSO₄ is +2.
- It can be oxidized to MnO₄⁻ (permanganate ion), where the oxidation state of Mn is +7.
- Hence, H₂O₂ can oxidize MnSO₄.
- The oxidation state of iron (Fe) in ferrous ion is +2.
- It can be oxidized to ferric ion (Fe³⁺), where the oxidation state of Fe is +3.
- Thus, H₂O₂ can oxidize Fe²⁺.
- Ozone is a powerful oxidizing agent itself.
- Since H₂O₂ is less powerful than ozone, it cannot oxidize ozone. Instead, ozone can act as an oxidizing agent and may reduce H₂O₂.
- Therefore, H₂O₂ cannot oxidize O₃.
- The substance that cannot be oxidized by H₂O₂ is Option D: Ozone (O₃).
Option D: Ozone (O₃) cannot be oxidized by H₂O₂.
Topper's Solved these Questions
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 472 Videos
- CHEMISTRY AT A GLANCEBook:ALLENChapter:CHEMISTRY AT A GLANCEExercise:ORGANIC CHEMISTRY
Explore 472 Videos
- Chemical EquilibriumBook:ALLENChapter:Chemical EquilibriumExercise:All Questions
Explore 30 Videos
- ELECTROCHEMISTRYBook:ALLENChapter:ELECTROCHEMISTRYExercise:EXERCISE -05 [B]
Explore 38 Videos
Similar Questions
Knowledge Check
Which of the following alcohols can be oxidised by K2Cr2O7?
ACH3−CH2−OHB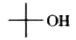 C
C DH2C=CH−CH2−OH
DH2C=CH−CH2−OHWhich of the following cannot be used as a test for H2O2?
AA paper dipped in Pbs (black) true white when brought in contact with H2O2.BIt liberates iodine from KI solution which gives blue color with starch solution.CIt gives blue color with K4[Fe(CN)6].DIt decolourises acidified KMnO4 solution.
Which of the following will not be oxidised by O3?
View SolutionWhich of the following is not oxidised by O3?
View SolutionWhich one of the following compounds would not be easily oxidised by K2Cr2O7in dilH2SO4 ?
View SolutionWhich oxide cannot be reduced by H2?
View SolutionAn organic compound (A) (C5H7OCl) reacts rapidly with ethanol to give (B) (C7H12O2). (A) also reacts with water to produce an acid which reacts with bromine to give (C ) (C5H8Br2O2). (B) on boiling with H2SO4 forms an acid (D). When (D) is oxidised with KMnO4 an acid (E ) (C4H6O3) is produced. On mild heating, (E ) gives (F) (C3H6O) which cannot be oxidised by ammoniacal AgNO3. Identify the compounds (A) to (F).
View SolutionCalculate the mass of oxalic acid (H2C2O4) which can be oxidised to CO2 by 100.0mL of MnO−4 solution, 10mL of which is capable of oxidising 50.0mL of 1.0NI− to I2?
View Solution
