When water is boiled at 2 atm pressure the latent heat of vaporization is 2.2×106J/kg and the boiling point is 120∘C At 2 atm pressure 1 kg of water has a volume of 10−3m−3 and 1 kg of steam has volume of 0.824m3 . The increase in internal energy of 1 kg of water when it is converted into steam at 2 atm pressure and 120∘C is [1 atm pressure =1.013×105N/m2]
2.033J
2.033×106J
0.167×106J
2.267×106J
The correct Answer is:B
To find the increase in internal energy of 1 kg of water when it is converted into steam at 2 atm pressure and 120°C, we will follow these steps:
Step 1: Calculate the heat absorbed (Q) during vaporization
The heat absorbed during the phase change from water to steam can be calculated using the formula:
Q=m×L
where:
- m = mass of water = 1 kg
- L = latent heat of vaporization = 2.2×106J/kg
Substituting the values:
Q=1kg×2.2×106J/kg=2.2×106J
Step 2: Calculate the work done (W) during vaporization
The work done by the gas during the phase change can be calculated using the formula:
W=PΔV
where:
- P = pressure = 2atm=2×1.013×105N/m2=2.026×105N/m2
- ΔV = change in volume = Vsteam−Vwater
Given:
- Volume of 1 kg of water Vwater=10−3m3
- Volume of 1 kg of steam Vsteam=0.824m3
Calculating ΔV:
ΔV=0.824m3−10−3m3=0.823m3
Now substituting the values into the work done formula:
W=2.026×105N/m2×0.823m3
W≈1.669×105J
Step 3: Calculate the change in internal energy (ΔU)
Using the first law of thermodynamics:
ΔU=Q−W
Substituting the values we calculated:
ΔU=2.2×106J−1.669×105J
ΔU≈2.033×106J
Final Answer
The increase in internal energy of 1 kg of water when it is converted into steam at 2 atm pressure and 120°C is approximately:
ΔU≈2.033×106J
---
The heat absorbed during the phase change from water to steam can be calculated using the formula:
Q=m×L
where:
- m = mass of water = 1 kg
- L = latent heat of vaporization = 2.2×106J/kg
Q=1kg×2.2×106J/kg=2.2×106J
The work done by the gas during the phase change can be calculated using the formula:
W=PΔV
where:
- P = pressure = 2atm=2×1.013×105N/m2=2.026×105N/m2
- ΔV = change in volume = Vsteam−Vwater
- Volume of 1 kg of water Vwater=10−3m3
- Volume of 1 kg of steam Vsteam=0.824m3
ΔV=0.824m3−10−3m3=0.823m3
W=2.026×105N/m2×0.823m3
W≈1.669×105J
Using the first law of thermodynamics:
ΔU=Q−W
ΔU=2.2×106J−1.669×105J
ΔU≈2.033×106J
The increase in internal energy of 1 kg of water when it is converted into steam at 2 atm pressure and 120°C is approximately:
ΔU≈2.033×106J
Topper's Solved these Questions
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:EXERCISE -01
Explore 64 Videos
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:EXERCISE -02
Explore 82 Videos
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:subjective
Explore 14 Videos
- CURRENT ELECTRICITYBook:ALLENChapter:CURRENT ELECTRICITYExercise:EX.II
Explore 66 Videos
- GRAVITATIONBook:ALLENChapter:GRAVITATIONExercise:EXERCISE 4
Explore 9 Videos
Similar Questions
On an X temperature scale, water freezes at −125.0∘X and boils at 375.0∘X. On a Y temperature scale, water freezes at −70.0∘Y and boils at −30.0∘Y. The value of temperature on X-scale equal to the temperature of 50.0∘Y on Y-scale is
View SolutionA triangular plate has two cavities , one square and one rectangular as shown in figure . The plate is heated.
_E01_066_Q01.png)
View SolutionA prism of refractive index n1 & another prism of reactive index n2 are stuck together without a gap as shown in the figure.The angle of the prisms are as shown. n1&n2depend onλ,the wavelength of light according to n1=1.20+10.8×104λ2&n2=1.45+1.80×104λ2 where λ is in nm.
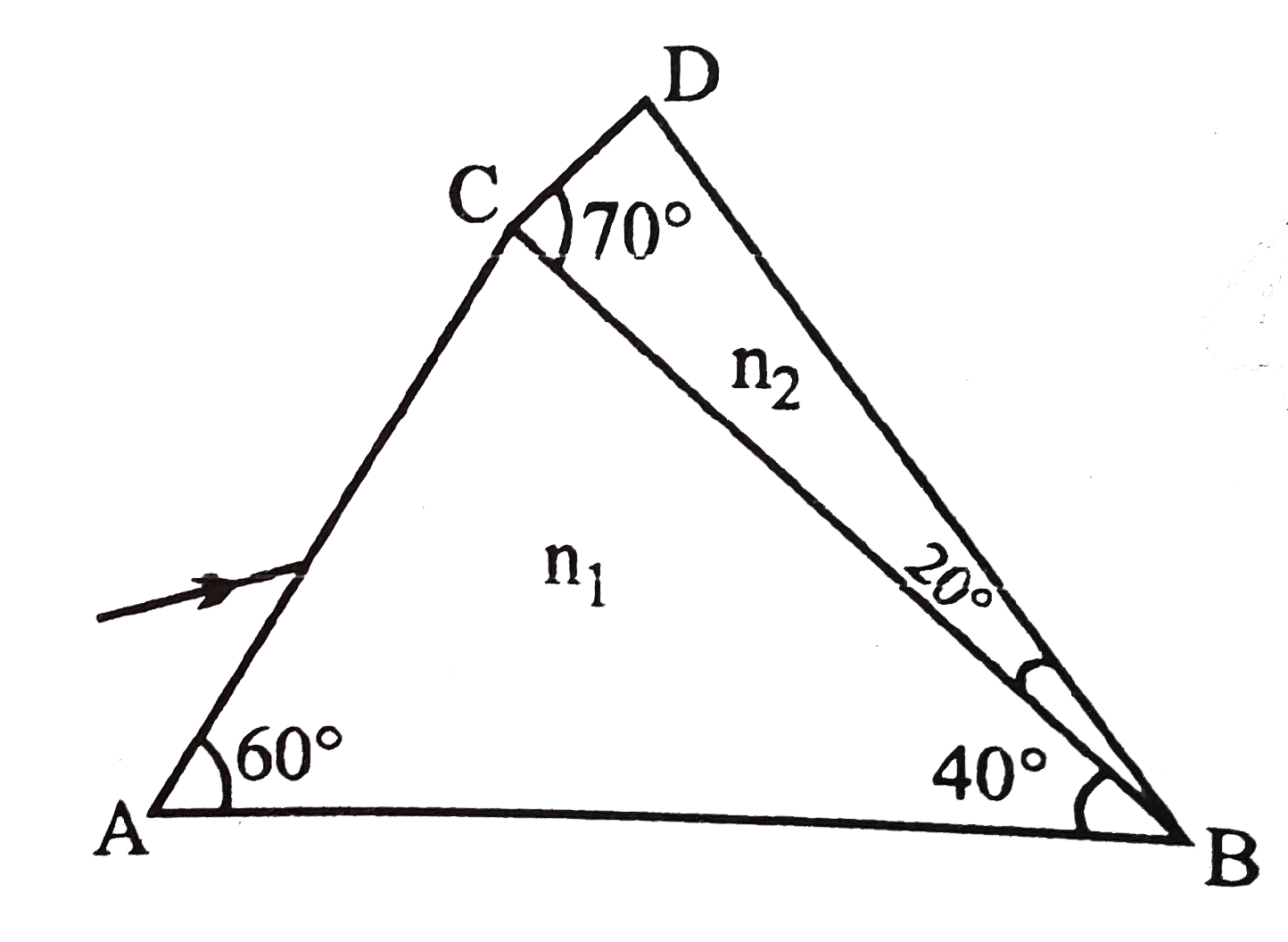
(i)Calculate the wavelength λ0for which rays incident at any angle on the interfaceBCpass through without bending at that interface.
View SolutionIn the Bohr's model of hy drogen atom, the electron moves around the nucleus in a circular orbit of radius 5×10−11m. Its time period is 1.5×10−16s. The current associated with the electron motion is:-
View SolutionAssertion : The difference in the value of acceleration due to gravity at pole and equator is proportional to square of angular velocity of earth.
Reason : the value of acceleration due to gravity is minimum at the equator and maximum at the pole.
View Solutioncalculate the increase in internal energy of 1 kg of water at 100(0)C when it is converted into steam at the same temperature and at 1atm (100 kPa). The density of water and steam are 1000 kg m−3 and 0.6 kg m−3 respectively. The latent heat of vaporization of water =2.25×106Jkg1.
View SolutionOne gram of water (1cm3) becomes 1671cm3 of steam when boiled at a constant pressure of 1 atm (1.013×105Pa). The heat of vaporization at this pressure is Lv=2.256×106J/kg. Compute (a) the work done by the water when it vaporizes and (b) its increase in internal energy.
View SolutionAt 100^(@)C the volume of 1 kg of water is 10^(-3) m^(3) and volume of 1 kg of steam at normal pressure is 1.671 m^(3) . The latent heat of steam is 2.3 xx 10^(6) J//kg and the normal pressure is 10^(5) N//m^(2) . If 5 kg of water at 100^(@)C is converted into steam, the increase in the internal energy of water in this process will be
View SolutionCalculate the increase in internal energy of 1 kg of water at 100∘C when it is converted into steam at the same temperature and at 1 atm (100kPa). The density of water and steam are 1000kgm3 and 0.6kgm3 resprectively.
The latent heat of vaporization of water =2.25×106Jkg
View SolutionThe increase in internal energy of 1 kg of water at 100∘ C when it is converted into steam at the same temperature and 1 atm (100 kPa) will be :
[The density of water and steam are 1000kg/m3and0.6kg/m3 respectively. The latent heat of vapourisation of water is 2.25×106J/kg.]
View SolutionWhat is the free energy change (DeltaG) when 1.0 "mole" of water at 100^(@)C and atm pressure is converted into steam at 100^(@)C and 1 atm pressure?
View SolutionWhat is the free energy change (ΔG) when 1.0mole of water at 100∘C and atm pressure is converted into steam at 100∘C and 1 atm pressure?
View Solution1cm3 of water at 1 atm pressure is converted into steam, volume of steam becomes 1671cm3 hence increase in internal energy will be ____
View Solutioncalculate the increase in internal energy of 1 kg of water at 100oC when it is converted into steam at the same temperature and at 1atm (100 kPa). The density of water and steam are 1000 kg m−3 and 0.6 kg m−3 respectively. The latent heat of vaporization of water =2.25×106Jkg1.
View SolutionAt atmospheric pressure, 2 g of water having a volume of 2.00cm becomes 3342cm3 of steam when boiled. The latent heat of vaporization of water is 539 cal/g at 1 atm. What is the amount of hat added to the system?
View SolutionA sample of 0.5 g water at 100°C and 1 atm pressure requires 270 cal heat energy to convert to steam at 100°C. If at the same pressure, volume of the steam produced is 835.5 cc then change in internal energy of the sample is (1 cal = 4.2 J, 1 atm 105Nm2)
View Solution
