Figure shows the variations of the internal energy U With density ρ one mole an ideal monatomic gas for thermodynamic cycle ABCA . Here process AB is a part of rectangular hyperbola,-
_S01_092_Q01.png)
process AB is isothermal & net work in cycle is done by gas.
process AB is isobaric & net work in cycle is done by gas.
process AB is isobaric & net work in cycle is done on the gas.
process AB is adiabatic & net work in cycle is done by gas.
The correct Answer is:B
For the process AB : Uρ = constant (hyperbola )
U=32RT (monoatomic iddeal gas), 32ρRT = constant
Comparing it with ideal gas equation P=(1M)ρRT⇒P is constant.
P−V graph for the cycle is . Thus work done in cycle positive
_S01_092_S01.png)
टॉपर्स ने हल किए ये सवाल
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:EXERCISE -01
Explore 64 Videos
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:EXERCISE -02
Explore 82 Videos
- GEOMETRICAL OPTICSBook:ALLENChapter:GEOMETRICAL OPTICSExercise:subjective
Explore 14 Videos
- CURRENT ELECTRICITYBook:ALLENChapter:CURRENT ELECTRICITYExercise:EX.II
Explore 66 Videos
- GRAVITATIONBook:ALLENChapter:GRAVITATIONExercise:EXERCISE 4
Explore 9 Videos
समान प्रश्न
Figure shows the variation of the internal energy U with the density ρ of an ideal monoatomic gas for a therodynamic process AB. Process AB is a part of arectangular hyperbola. Find the work done (in joule) by gas in process AB.
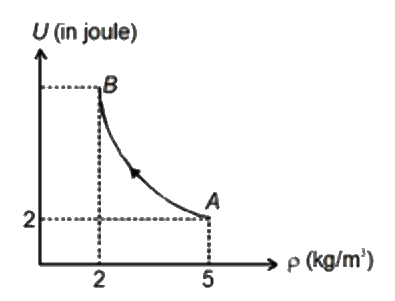
View Solution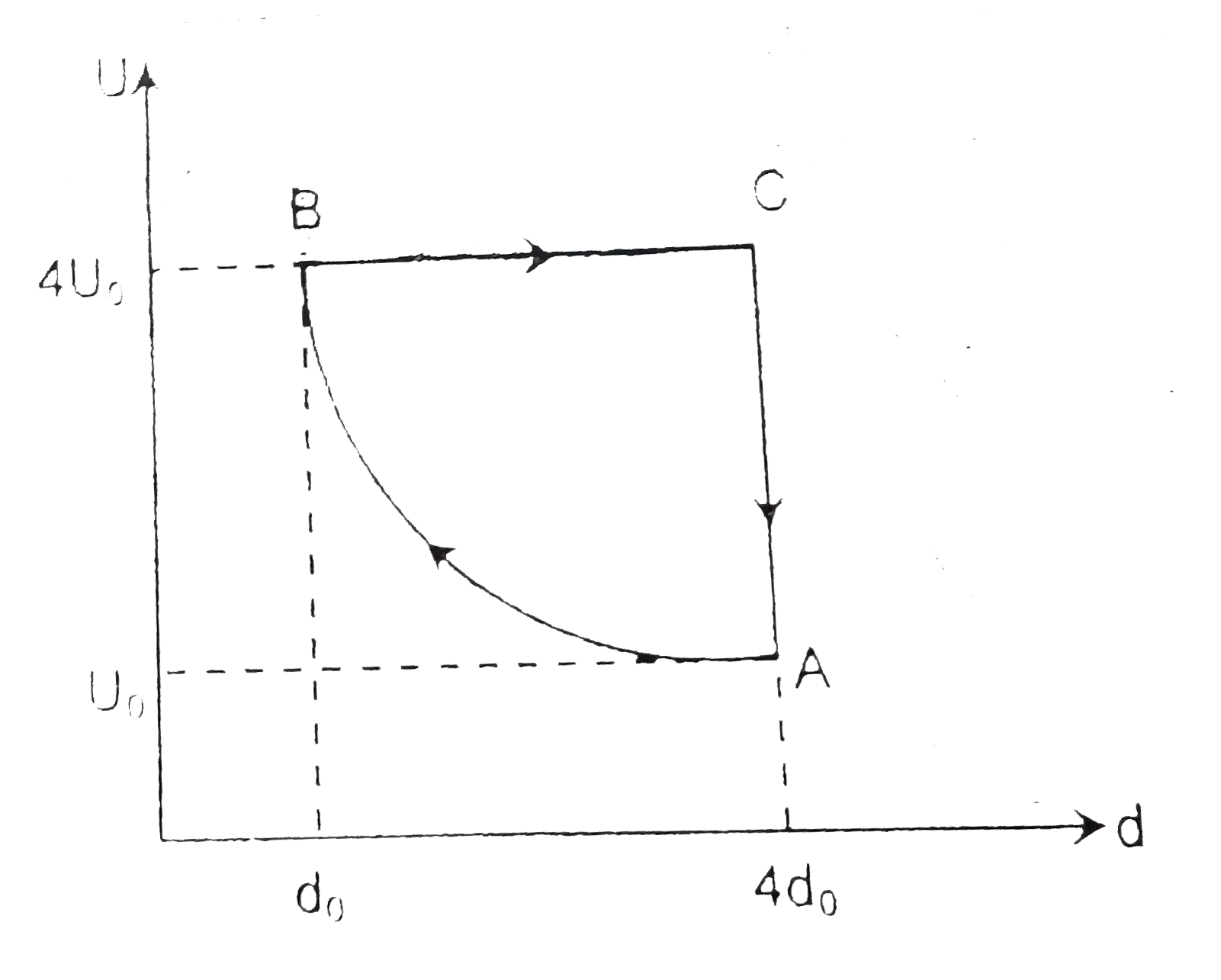
The variation of internal energy U and density d of one mole of an ideal monatomic gas is shown inn cyclic process ABCA. The curve from A to B is a rectangular hyperbola. Select correct alternatives.
View Solution
ज्ञान जांच
A thermodynamic process of one mole ideal monoatomic gas is shown in figure. The efficiency of cyclic process ABCA will be
A0.25B0.125C0.5D0.077
One mole of an ideal monoatomatic gas is taken round the cylic process ABCA as shown Then
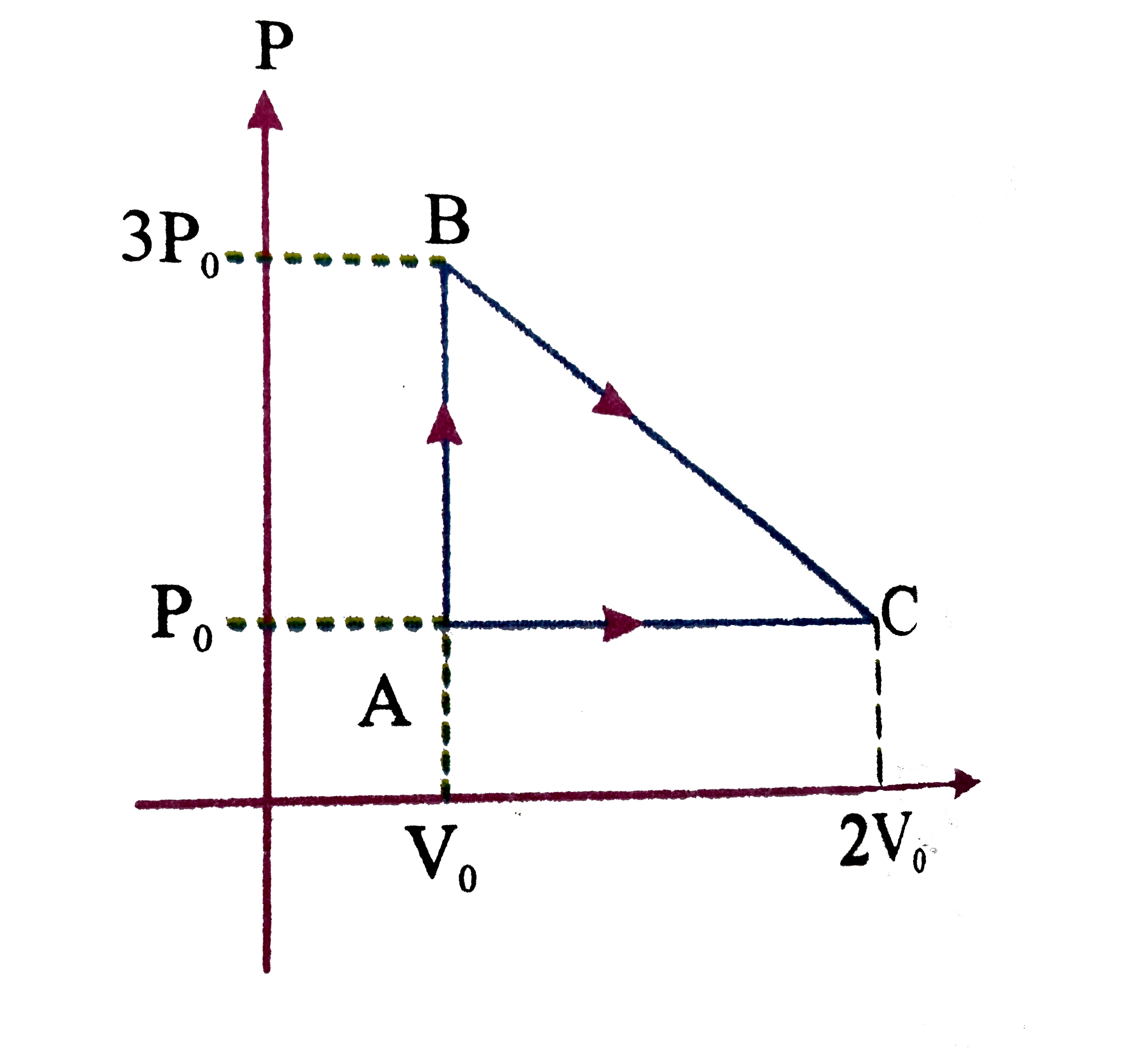
View SolutionThe internal energy of one mole of ideal gas is
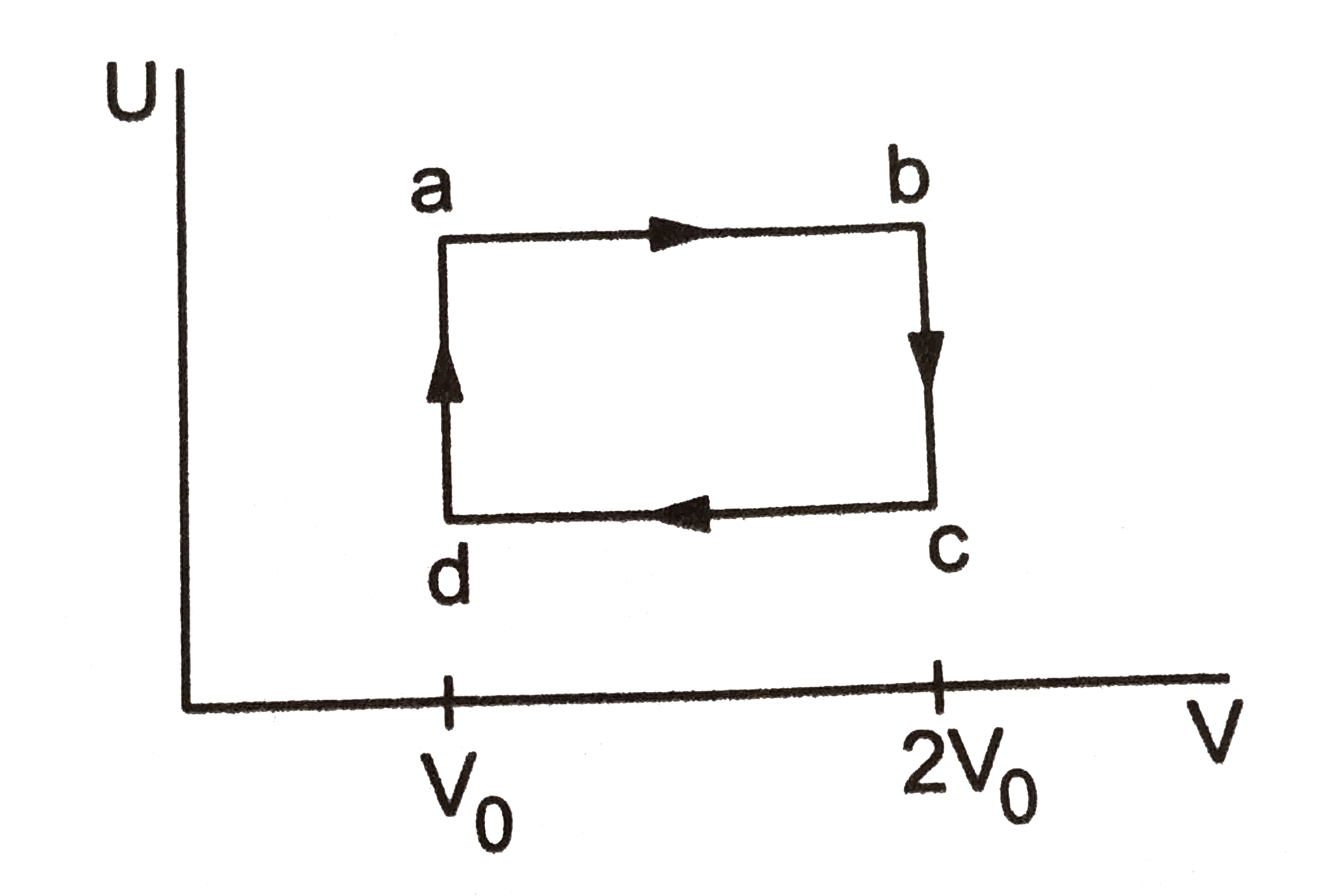
View SolutionFig shows the variation in the internal energy U with the volume V of 2.0 mol of an ideal gas in a cyclic process abcda. The temperatures of the gas at b and c are 500 K and 300 K respectively. Calculate the heat absorbed by the gas during the process.
View SolutionThe given graph shows variation (with distance r form center) of :
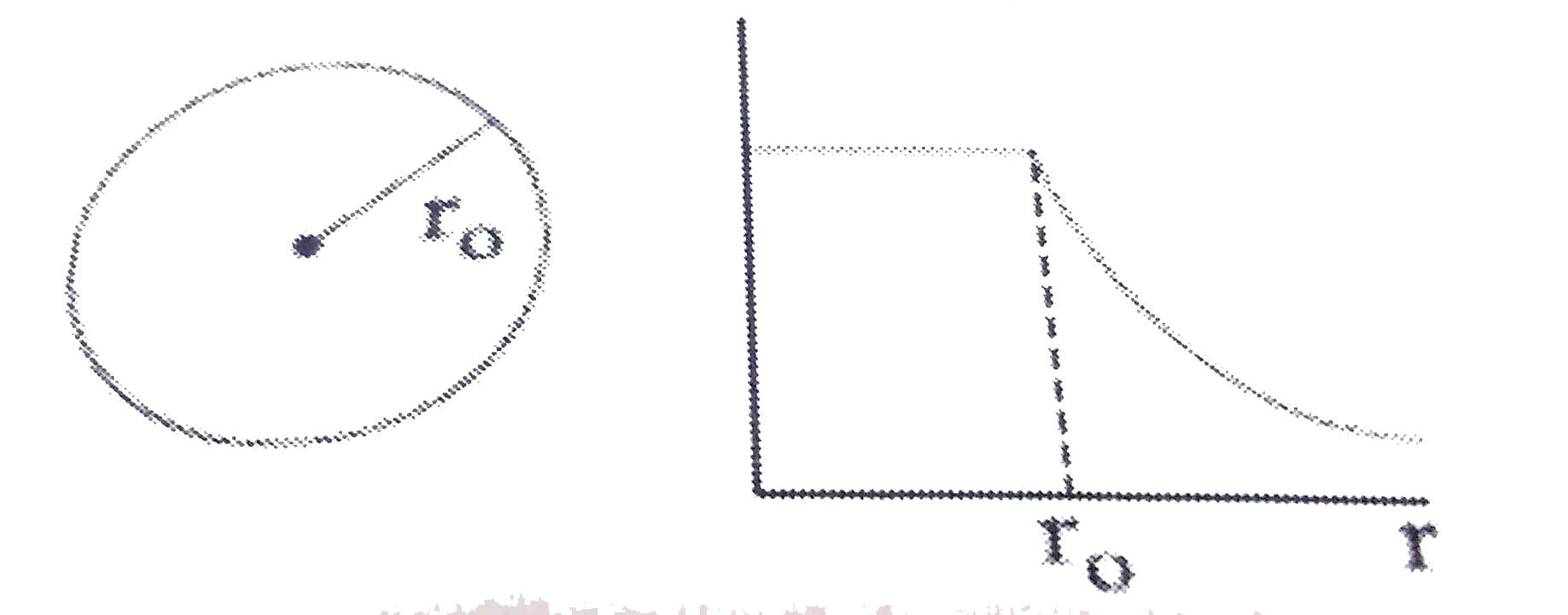
View SolutionWhich one is not correct for a cyclic process as shown in the figure ?
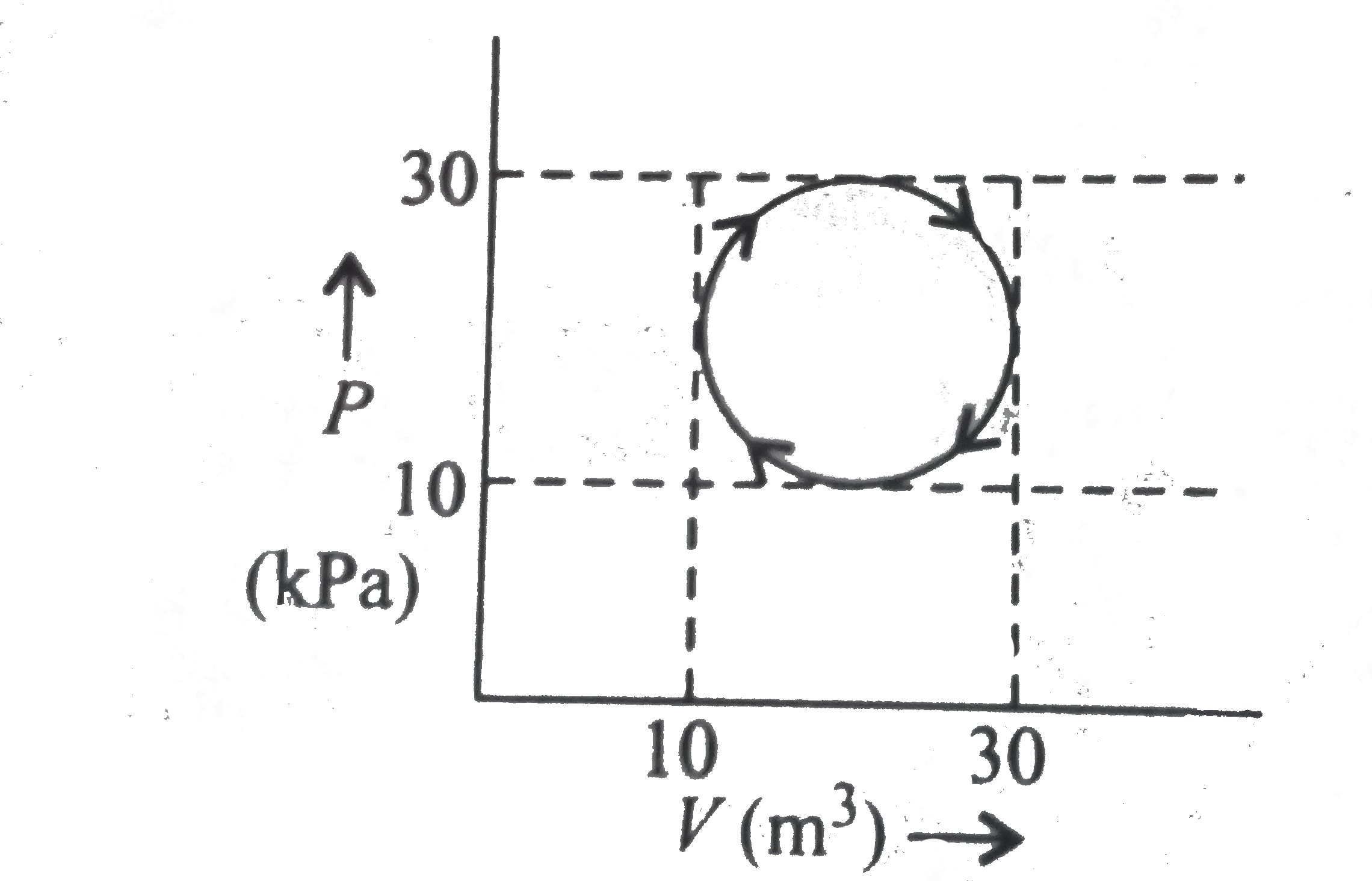
View SolutionA thermodynamic process of one mole ideal monoatomic gas is shown in figure. The efficiency of cyclic process ABCA will be

View SolutionFigure, shows the variation of potential energy (U) of 2 mol of Argon gas with its density in a cyclic process ABCA. The gas was initiallly in the state A whose pressure and temperature are PA=2atm,TA=300K, respectively. It is also stated the path AB is a rectangular hyperbola and the internal energy of the gas at state C is 3000r. Based on the above information answer the following question :
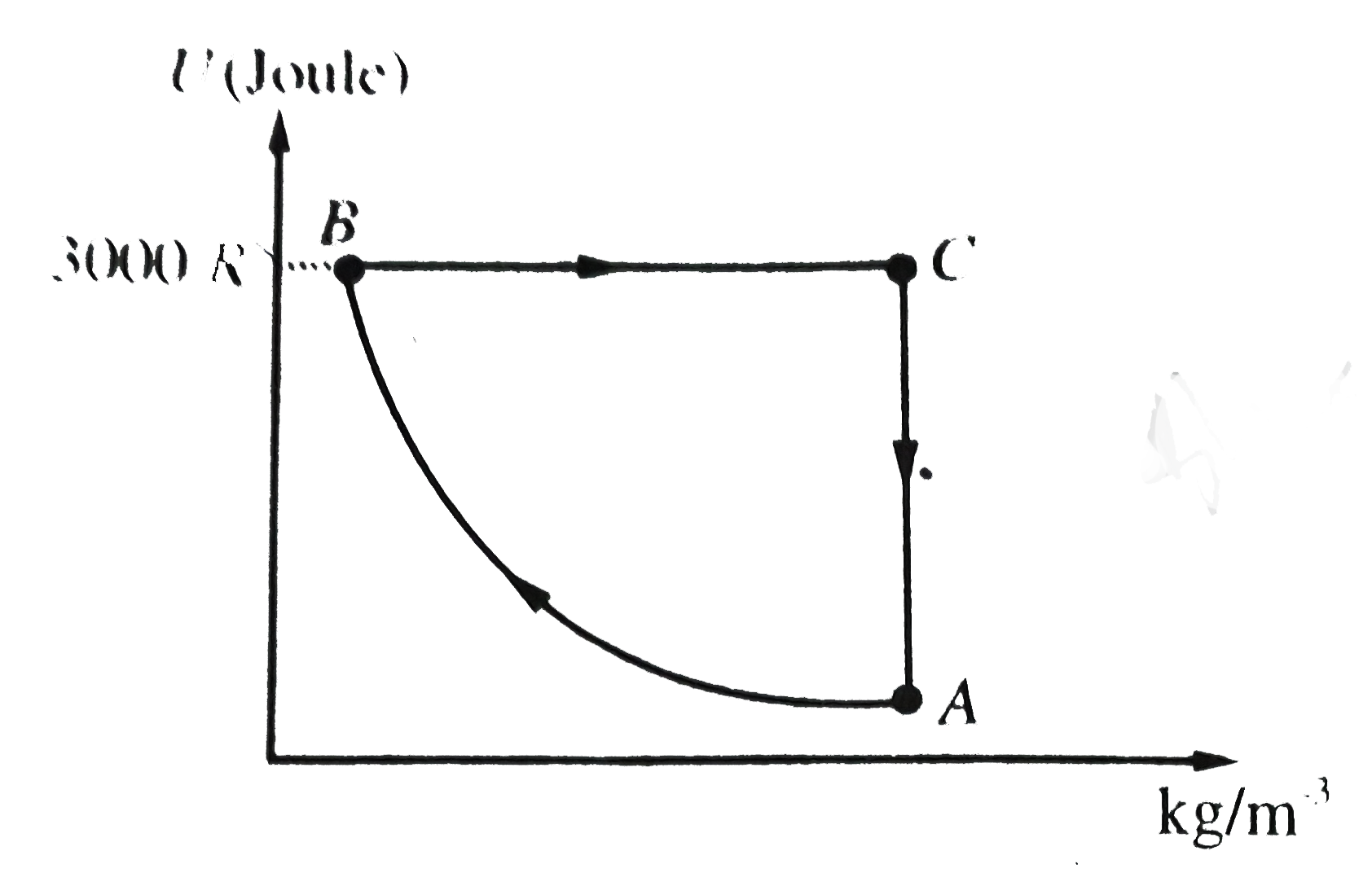
The heat supplied to the gas in the process AB is
View Solution
