Titanium , which is used to make air plane engines and frames, can be obtained from titanium tetrachloride , which in turn is obtained from titanium oxide by the following process :
3TiO2(s)+4C(s)+6Cl2(g)→3TiCl4(g)+2CO2(g)+2CO(g)
A vessel contains 4.3 g TiO2,5.76gCand,7.1gCl2 ,suppose the reaction goes to completion as written , how many gram of TiCl4 can be produced ? (Ti =48)
The correct Answer is:9.5
To solve the problem of how many grams of titanium tetrachloride (TiCl₄) can be produced from the given amounts of titanium dioxide (TiO₂), carbon (C), and chlorine (Cl₂), we will follow these steps:
Step 1: Write the balanced chemical equation
The balanced chemical equation for the reaction is:
3TiO2(s)+4C(s)+6Cl2(g)→3TiCl4(g)+2CO2(g)+2CO(g)
Step 2: Calculate the molar masses of the reactants
- Molar mass of TiO₂:
- Titanium (Ti) = 48 g/mol
- Oxygen (O) = 16 g/mol
- Molar mass of TiO₂ = 48 + (2 × 16) = 48 + 32 = 80 g/mol
- Molar mass of C (Carbon) = 12 g/mol
- Molar mass of Cl₂ (Chlorine) = 2 × 35.5 = 71 g/mol
Step 3: Calculate the number of moles of each reactant
- Moles of TiO₂:
Moles of TiO2=4.3 g80 g/mol=0.05375 moles
- Moles of C:
Moles of C=5.76 g12 g/mol=0.48 moles
- Moles of Cl₂:
Moles of Cl2=7.1 g71 g/mol=0.1 moles
Step 4: Determine the stoichiometric ratios
From the balanced equation:
- 3 moles of TiO₂ react with 4 moles of C and 6 moles of Cl₂.
Now, we will calculate the ratio of moles of each reactant to its stoichiometric coefficient:
- For TiO₂:
0.053753=0.01792
- For C:
0.484=0.12
- For Cl₂:
0.16=0.01667
Step 5: Identify the limiting reagent
The limiting reagent is the one with the smallest ratio:
- TiO₂: 0.01792
- C: 0.12
- Cl₂: 0.01667
Thus, Cl₂ is the limiting reagent.
Step 6: Calculate the amount of TiCl₄ produced
From the balanced equation, 6 moles of Cl₂ produce 3 moles of TiCl₄. Therefore:
- Moles of TiCl₄ produced from 0.1 moles of Cl₂:
Moles of TiCl4=0.1×36=0.05 moles
Step 7: Calculate the mass of TiCl₄ produced
- Molar mass of TiCl₄:
Molar mass of TiCl4=48+(4×35.5)=48+142=190 g/mol
- Mass of TiCl₄ produced:
Mass of TiCl4=Moles×Molar mass=0.05 moles×190 g/mol=9.5 g
Final Answer
The mass of TiCl₄ that can be produced is 9.5 grams.
---
The balanced chemical equation for the reaction is:
3TiO2(s)+4C(s)+6Cl2(g)→3TiCl4(g)+2CO2(g)+2CO(g)
- Molar mass of TiO₂:
- Titanium (Ti) = 48 g/mol
- Oxygen (O) = 16 g/mol
- Molar mass of TiO₂ = 48 + (2 × 16) = 48 + 32 = 80 g/mol
- Moles of TiO₂:
Moles of TiO2=4.3 g80 g/mol=0.05375 moles
Moles of C=5.76 g12 g/mol=0.48 moles
Moles of Cl2=7.1 g71 g/mol=0.1 moles
From the balanced equation:
- 3 moles of TiO₂ react with 4 moles of C and 6 moles of Cl₂.
- For TiO₂:
0.053753=0.01792
- For C:
0.484=0.12
- For Cl₂:
0.16=0.01667
The limiting reagent is the one with the smallest ratio:
- TiO₂: 0.01792
- C: 0.12
- Cl₂: 0.01667
From the balanced equation, 6 moles of Cl₂ produce 3 moles of TiCl₄. Therefore:
- Moles of TiCl₄ produced from 0.1 moles of Cl₂:
Moles of TiCl4=0.1×36=0.05 moles
- Molar mass of TiCl₄:
Molar mass of TiCl4=48+(4×35.5)=48+142=190 g/mol
Mass of TiCl4=Moles×Molar mass=0.05 moles×190 g/mol=9.5 g
The mass of TiCl₄ that can be produced is 9.5 grams.
Topper's Solved these Questions
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:S-II
Explore 18 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:O-I
Explore 45 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:Basic Exercise
Explore 29 Videos
- METALLURGYBook:ALLENChapter:METALLURGYExercise:EXERCISE-05 [B]
Explore 26 Videos
- p-Block Element Book:ALLENChapter:p-Block Element Exercise:All Questions
Explore 20 Videos
Similar Questions
Two substances P4 &O2 are allowed to react completely to form mixture of P4O6 &P4O10 leaving none of the reactants .Using this information calculate the moles of P4O6 and P4O10 in the final mixture when the following amounts of P4 & O2 are taken.
P4+3O2→P4O6
P4+5O2→P4O10
(i) If 1 mole P4 & 4 mole of O2
(ii) If 3 mole P4 & 11 mole of O2
(iii) If 3 mole P4 & 13 mole of O2
View SolutionOne atomic mass unit in kilogram is
View Solution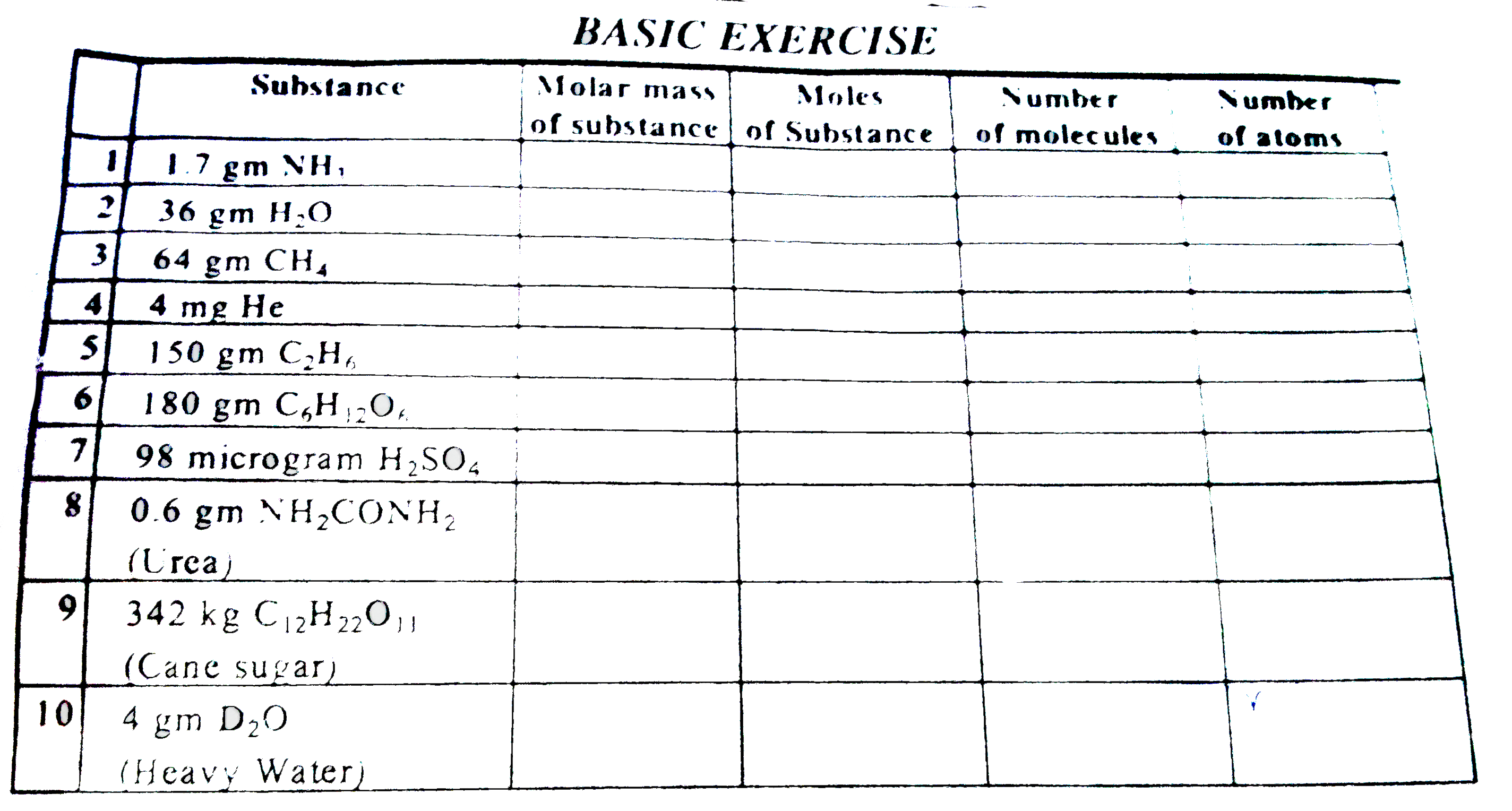
A box contains 12×1022 number of oxygen atoms. Find moles of O-atoms? (NA=6×1023)
View SolutionWhen the ore haematite is burnt in air with coke around 2000K along with lime, the process not only produces steel but also produces a silicate slag that is useful in making building materials such as cement. Discuss the same and show through balanced chemical equation.
View SolutionThe main factor responsible for weak acidic nature of BF3 is :-
View SolutionWhat would be the effect of increasing the volume of each of the following system at equilibrium?
a. 2CO(g)+O2(g)⇔2CO2(g)
b. NI(s)+4CO(g)⇔NI(CO)4(g)
c. N2O4(g)⇔2NO2(g)
View SolutionTitanium metal is extensively used in aerospace industry because the metal imparts strength to structures but does not unduly add to their masses. The metal is produced by the reduction of TiCl_(4(l)) which in turn is produced from mineral rutile [TiO_(2(s))] . can the following reaction for production of TiCl_(4(l)) be carried out at 25^(@)C ? TiO_(2(s))+2Cl_(2(g))toTiCl_(4(l))+O_(2(g)) Given that H_(f)^(@) for TiO_(2(s)),TiCl_(4(l)),Cl_(2(g)) and O_(2(g)) are -944.7, -804.2,0.0,0.0 kJ mol^(-1) . also S^(@) for TiO_(2(g)),TiCl_(4(l)),Cl_(2(g)) and O_(2(g)) are 50.3,252.3,233.0,205.1 J mol^(-1) K^(-1) respectively.
View SolutionTitanium oxide (TiO2) is heated with excess hydrogen gas to give water and nee oxide TixOy. If 1.6gmTiO2 produces 1.44gTixOy, then select statement (Molar mass of titanium is 48g/mol):
View SolutionPure PCl5(g) was introduced into an evacuated vessel and comes to equilibrium at 24∘C and 2 atm pressure. At equilibrium mixture contains 40% by volume of Cl2(g)
PCl5(g)⇔(PCl3(g)+Cl2(g)
Which of the following statement(s) regarding the above equilibrium is/are correct?
View SolutionCalculate DeltaH^(@) for the reaction : TiCl_(4)(g)+2H_(2)O(l)to TiO_(2)(s)+4Hcl(g) {:(DeltaH_(f)^(@),(KJ.mol,^(-1))),(TiCl_(4)(g),-763),(H_(2)O(l),-286),(TiO_(2)(s),-945),(HCl(g),-92):}
View SolutionAt high temperature the following equilibria exist in a mixture of carbon, oxygen and their compounds :
C(s)+O2(g)⇔CO2(g)K1=10−22C(s)+O2(g)⇔2CO(g)K2=10−3C(s)+CO2(g)⇔2CO(g)K3=x2CO(g)+O2(g)⇔2CO2(g)K4=y
What will the ratio of x & y?
View Solutionनिम्नलिखित में से दाब बढ़ने पर कौन-कौन से अभिक्रियाएँ प्रभावित होंगी? यह भी बताएँ की दाब परिवर्तन करने पर क्या अभिक्रिया अग्र या प्रतीप दिशा में गतिमान होगी ?
(i)COCI2(g)⇔CO(g)+CI2(g)
(ii) CH4(g)+2S2⇔CS2+2H2S(g)
(iii) CO2(g)+C(s)⇔2CO(g)
(iv)2H2(g)+CO(g)⇔CH3OH(g)
(v)CaCO3(s)⇔CaO(s)+CO2(g)
(vi) 4NH3(g)+5O2(g)⇔4NO(g)+6H2O(g)
View Solutionनिम्नलिखित में दाब बढ़ाने पर कौन- कौन सी अभिक्रियाएँ प्रभावित होगी ? यह भी बताएँ की दाब परिवर्तन करने पर अभिक्रिया अग्र या प्रतीप दिशा में गतिमान होगी ?
(i)COCl2(g)⇔CO(g)+Cl2(g)
(ii)CH4(g)+2S2(g)⇔CS2(g)+2H2S(g)
(iii)CO2(g)+C(s)⇔2CO(g)
(iv)2H2(g)+CO(g)⇔CH3OH(g)
(v)CaCO3(s)⇔CaO(s)+CO2(g)
(vi)NH3(g)+5O2(g)⇔4NO(g)+6H2O(g)
View SolutionWhich of the following reactions will get affected by increasing the pressure? Also mention whether chasnge will cause the reaction to go into forward or backward direction. (i) COCl_(2)(g)hArrCO(g)+Cl_(2)(g) (ii) CH_(4)(g)+2S_(2)(g)hArrCS_(2)(g)+2H_(2)S(g) (iii) CO_(2)(g)+C(s)hArr2CO(g) (iv) 4NH_(3)+(g)+5O_(2)(g)hArr4NO(g)+6H_(2)O(g)
View Solutionनीचे दी गयी अभिक्रियाओं में पहचानिए कि किसका ऑक्सीकरण हो रहा है और किसका अपचयन ?
H2S(s)+Cl2(g)→2HCl(g)+S(s)
View Solution3.24 g of titanium reacts with oxygen to form 5.40 g of the metal oxide. Find the empirical formula of the metal oxide ?
View Solution
