In the reaction sequence
RCOCl+H2Pd+BaSO4−−−−−−→AHCN−−→BH3O⊕−−−→C
A,B and C are given by the set :-
RCHO,RCH(OH)CN,RCH(OH)CH2NH2
RCHO,RCH(OH)CN,RCH(OH)COOH
RCHO,R−C∣∣O−C∣CNH2,R−C∣∣O−OH
RCHO,R−CH2−CN,R−CH2−COOH
The correct Answer is:B
To solve the given reaction sequence, we will analyze each step carefully.
1. Identify the Starting Material:
The starting material is RCOCl (an acyl chloride).
2. First Reaction:
The first reaction involves RCOCl reacting with H2 in the presence of Pd/BaSO4. This is a Rosenmund reduction, which converts acyl chlorides to aldehydes.
- Product A: The product of this reaction is RCHO (an aldehyde).
3. Second Reaction:
The aldehyde (RCHO) then reacts with HCN (hydrogen cyanide). In this reaction, the carbonyl carbon of the aldehyde is attacked by the cyanide ion (CN⁻), leading to the formation of a cyanohydrin.
- Product B: The product of this reaction is RCH(OH)CN (the cyanohydrin).
4. Third Reaction:
The cyanohydrin (RCH(OH)CN) is then treated with H3O⁺ (acidic conditions). Under acidic conditions, the cyanohydrin undergoes hydrolysis, converting the cyano group (CN) into a carboxylic acid (COOH).
- Product C: The final product is RCOOH (a carboxylic acid).
Summary of Products:
- A: RCHO (aldehyde)
- B: RCH(OH)CN (cyanohydrin)
- C: RCOOH (carboxylic acid)
Final Answer:
The correct set of products A, B, and C is:
- A = RCHO
- B = RCH(OH)CN
- C = RCOOH
The starting material is RCOCl (an acyl chloride).
The first reaction involves RCOCl reacting with H2 in the presence of Pd/BaSO4. This is a Rosenmund reduction, which converts acyl chlorides to aldehydes.
- Product A: The product of this reaction is RCHO (an aldehyde).
The aldehyde (RCHO) then reacts with HCN (hydrogen cyanide). In this reaction, the carbonyl carbon of the aldehyde is attacked by the cyanide ion (CN⁻), leading to the formation of a cyanohydrin.
- Product B: The product of this reaction is RCH(OH)CN (the cyanohydrin).
The cyanohydrin (RCH(OH)CN) is then treated with H3O⁺ (acidic conditions). Under acidic conditions, the cyanohydrin undergoes hydrolysis, converting the cyano group (CN) into a carboxylic acid (COOH).
- Product C: The final product is RCOOH (a carboxylic acid).
- A: RCHO (aldehyde)
- B: RCH(OH)CN (cyanohydrin)
- C: RCOOH (carboxylic acid)
The correct set of products A, B, and C is:
- A = RCHO
- B = RCH(OH)CN
- C = RCOOH
Topper's Solved these Questions
- ALKENE, ALKANE & ALKYNE BOOK - ALLENCHAPTER - ALKENE, ALKANE & ALKYNE EXERCISE - EXERCISE-2
103 Videos
- ALKENE, ALKANE & ALKYNE BOOK - ALLENCHAPTER - ALKENE, ALKANE & ALKYNE EXERCISE - EXERCISE-3
99 Videos
- ALKENE, ALKANE & ALKYNE BOOK - ALLENCHAPTER - ALKENE, ALKANE & ALKYNE EXERCISE - EXCERSISE-3
28 Videos
- AIIMS 2019BOOK - ALLENCHAPTER - AIIMS 2019EXERCISE - CHEMISTRY
40 Videos
- ALKYL AND ARYL HALIDEBOOK - ALLENCHAPTER - ALKYL AND ARYL HALIDEEXERCISE - EXERCISE-05 (B)
35 Videos
Similar Questions
Knowledge Check
In the given the reaction sequence
C6H5CHOH2N−OH−−−−−−−−→H⊕,ΔAP2O5−−→B
A and B respectively are:AC6H5−CH=N−OH,C6H5CNBC6H5−CH=N−OH,C6H5O∣∣C−NH2CC6H5−CH=N−OH,C6H5CHODC6H5−CH=N−OH,C6H5−COOH
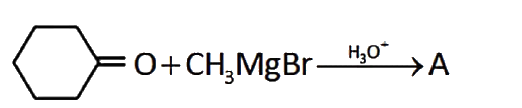
Conc. H2SO4−−−−−−−−→BO3/H2O/Zn−−−−−−−→C. A, B and C are -
View SolutionFind the end product in the given sequence of reaction HC≡CH1%HgSO4−−−−−−→ACH3MgX−−−−−→B[O]−−→
View SolutionName the reaction sequence.
RCOOHSOCl2−−−→RCOClCH2N2−−−→RCOCHN2H2O−−→AgRCH2COOH.
View SolutionIn the reaction sequence C6H5CHONaCN/HCl−−−−−−−→(X)H2O/H⊕−−−−−→ product, Product will be
View SolutionComplete the following :
C6H5COCl+H2Pd/BaSO4−−−−−−→
View SolutionCompounds A, B and C in the following reaction sequence are:
C2H5BrAgCN−−−→AH3O+−−−→ B + C
View SolutionIn the reaction sequence :- ASnCl2/HCl−−−−−−→BNaNO2/HCl−−−−−−−→0∘CH2O/Δ−−−−→C6H5OH, A,B and C are :-
View Solution

