If each orbital can hold a maximum of 3 electrons the number of elements in 2nd period of periodic table ( long form ) is
8
12
18
24
The correct Answer is:B
Step by step video solution for If each orbital can hold a maximum of 3 electrons the number of elements in 2 ^(nd) period of periodic table ( long form ) is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
In the following compounds
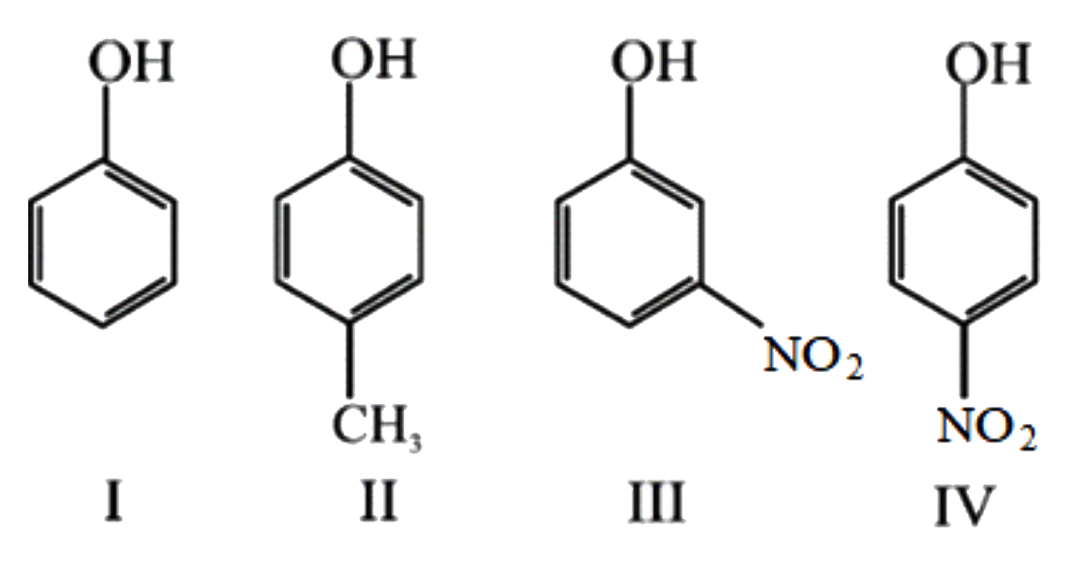
The order of acidity is
View Solution_E01_735_Q01.png) True or False
True or False
View SolutionIt each orbital can hold a maximum of three electrons, the number of elements in 9th period of periodic table (long form) are:
View SolutionIf each orbital can hold a maximum of 3 electrons, the number of elements in 4th periodic table (long form) is.
View SolutionIn the long form of periodic table __________ group contains the maximum number of elements.
View SolutionIn the long form of the periodic table, ________
group elements can release maximum amount of
energy by the addition of electron into is valence
shell.
View SolutionThe number of elements in each of the long periods in the periodic table is
View SolutionIf each orbital can hold a maximum of 3 electrons. Then the number of elements in 2 ""^(nd) period of the periodic table is :
View Solutionआवर्त सारणी के प्रत्येक दीर्घ आवर्त की आवर्त में तत्वों की संख्या होती है
View SolutionAssertion : 2nd period elements do not involve in excitation of electron.
Reason : 2nd period elements do not have vacant 2d-orbitals.
View SolutionIf each orbital can accommodate maximum of four electrons,, the number of elements in the third period of the periodic table will be-
View SolutionNumber of elements present in period - 2 of the long form periodic table _________
View SolutionIf each orbital can take maximum of three electrons, the number of elements in the third period of the periodic table will be:
View SolutionMention the number of elements present in each of the periods in the long form periodic table.
View SolutionIf each orbital can hold a maximum of 3-electrons. The number of elements in 4^(th) period of periodic table is :
View SolutionMention the number of elements present in each of the periods in the long form of table?
View Solution
