Which of the following Allene is achiral?
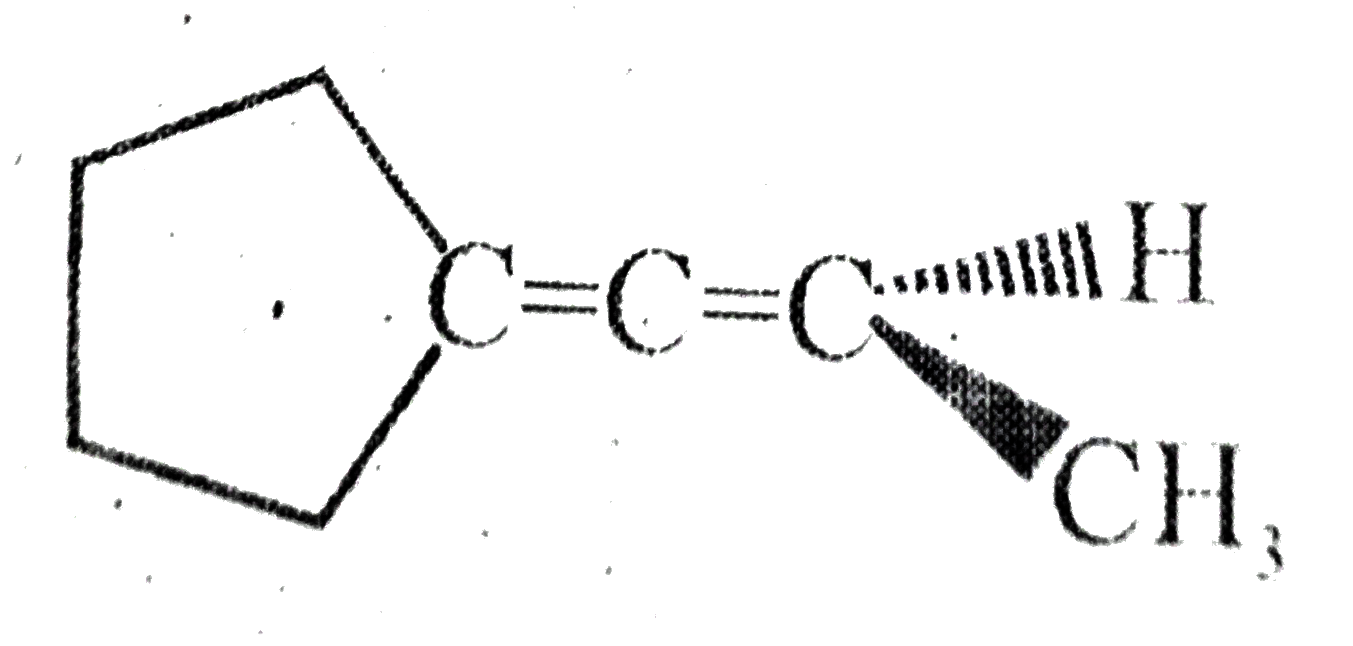
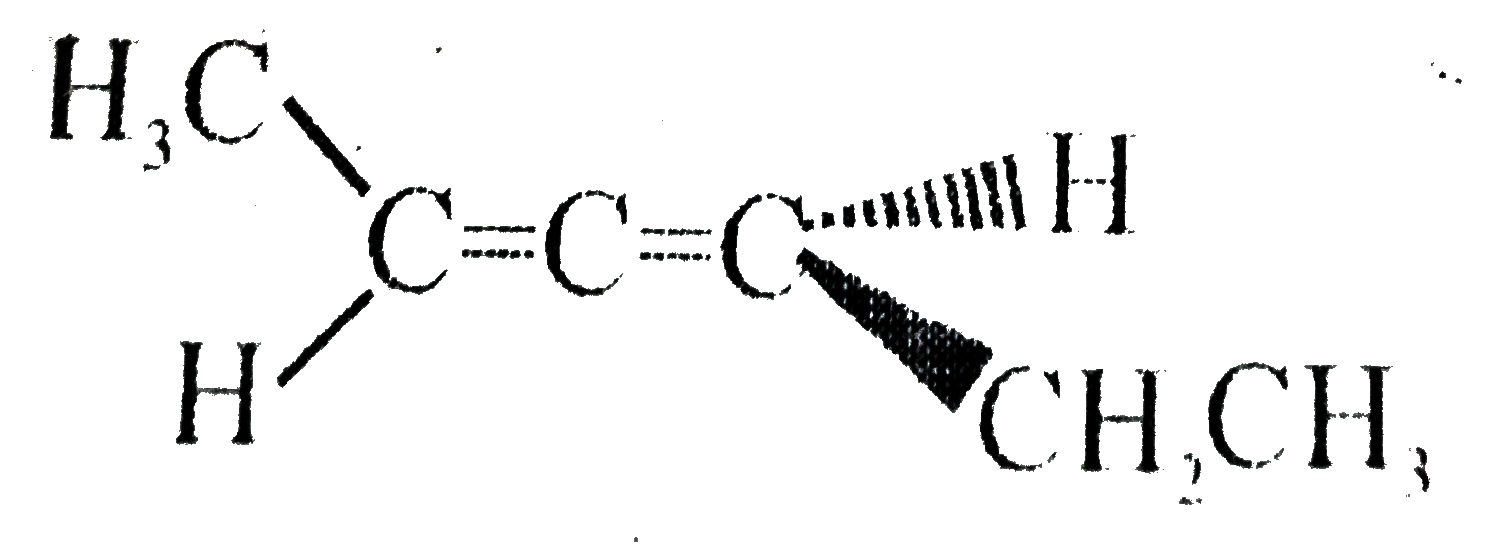
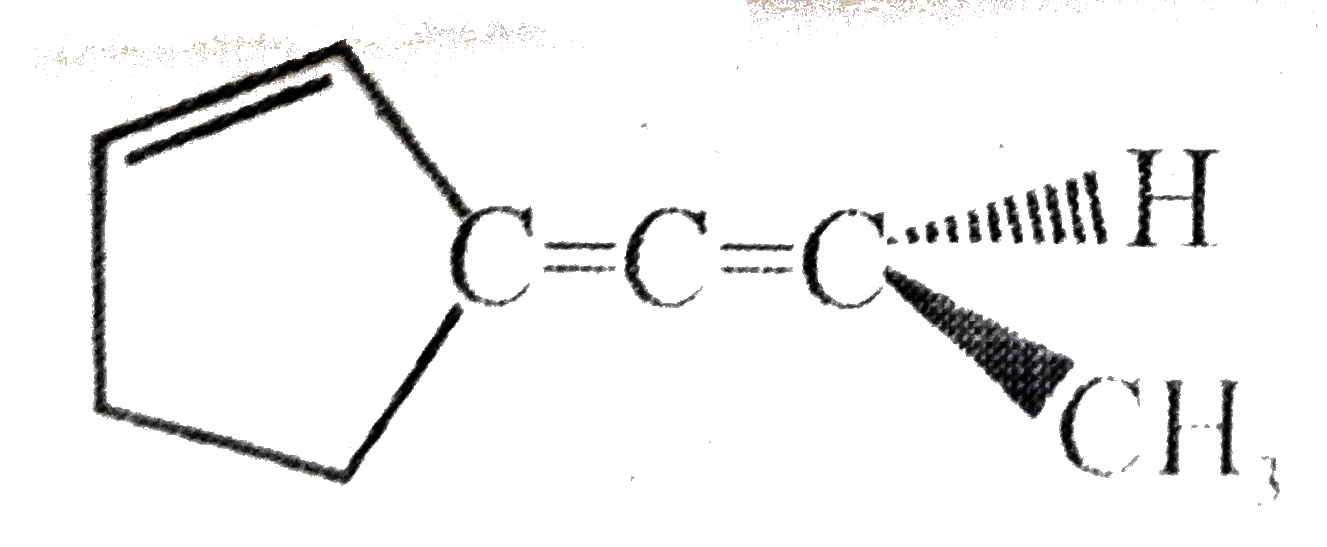
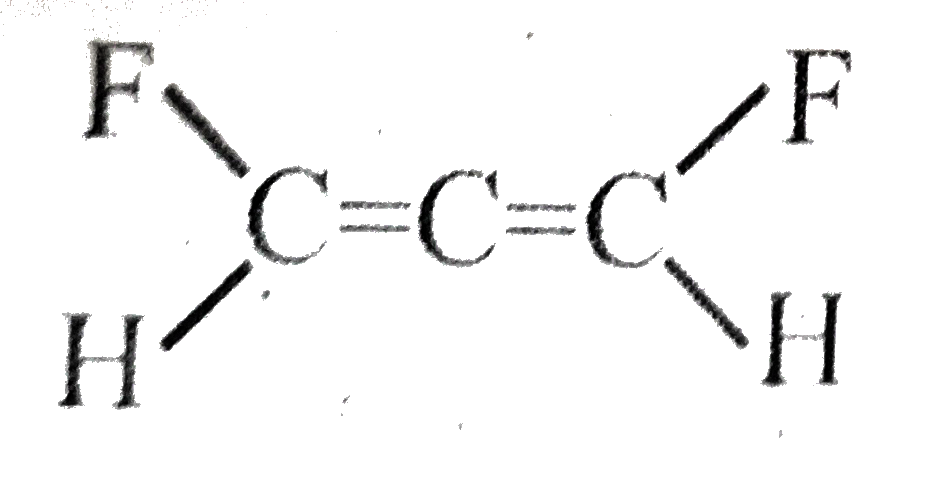
The correct Answer is:A
To determine which of the following allenes is achiral, we need to analyze the chirality of each compound based on the structural requirements for chirality.
1. Understanding Chirality:
- Chirality refers to a property of a molecule that makes it non-superimposable on its mirror image. For a carbon atom to be chiral, it must be sp³ hybridized and bonded to four different substituents.
2. Identifying the Structure of Allenes:
- Allenes have a cumulated double bond structure (C=C=C). In allenes, the central carbon atom is sp hybridized, while the terminal carbons are sp² hybridized.
3. Analyzing Each Allene:
- For each allene, we need to check if there are four different substituents around the sp² hybridized carbon atoms and if there is any symmetry.
4. Case Analysis:
- Allene 1: If it has two identical substituents on one side (e.g., H and CH₃ on one side, and H and H on the other), it is symmetric and thus achiral.
- Allene 2: If it has two identical substituents (e.g., H and H on one side, and C₂H₅ and H on the other), it is also symmetric and thus achiral.
- Allene 3: If it has four different substituents (e.g., H, CH₃, C₂H₅, and another different group), it is asymmetric and thus chiral.
- Allene 4: If it has two identical substituents (e.g., F and F on one side, and H and H on the other), it is symmetric and thus achiral.
5. Conclusion:
- After analyzing the structures, we find that the allenes with identical substituents on one side are achiral. Therefore, the answer to the question is that the achiral allene is the one with symmetry due to identical substituents.
Final Answer:
The achiral allene is the one that has symmetry due to identical substituents.
- Chirality refers to a property of a molecule that makes it non-superimposable on its mirror image. For a carbon atom to be chiral, it must be sp³ hybridized and bonded to four different substituents.
- Allenes have a cumulated double bond structure (C=C=C). In allenes, the central carbon atom is sp hybridized, while the terminal carbons are sp² hybridized.
- For each allene, we need to check if there are four different substituents around the sp² hybridized carbon atoms and if there is any symmetry.
- Allene 1: If it has two identical substituents on one side (e.g., H and CH₃ on one side, and H and H on the other), it is symmetric and thus achiral.
- Allene 2: If it has two identical substituents (e.g., H and H on one side, and C₂H₅ and H on the other), it is also symmetric and thus achiral.
- Allene 3: If it has four different substituents (e.g., H, CH₃, C₂H₅, and another different group), it is asymmetric and thus chiral.
- Allene 4: If it has two identical substituents (e.g., F and F on one side, and H and H on the other), it is symmetric and thus achiral.
- After analyzing the structures, we find that the allenes with identical substituents on one side are achiral. Therefore, the answer to the question is that the achiral allene is the one with symmetry due to identical substituents.
The achiral allene is the one that has symmetry due to identical substituents.
Topper's Solved these Questions
Similar Questions
In the following compounds
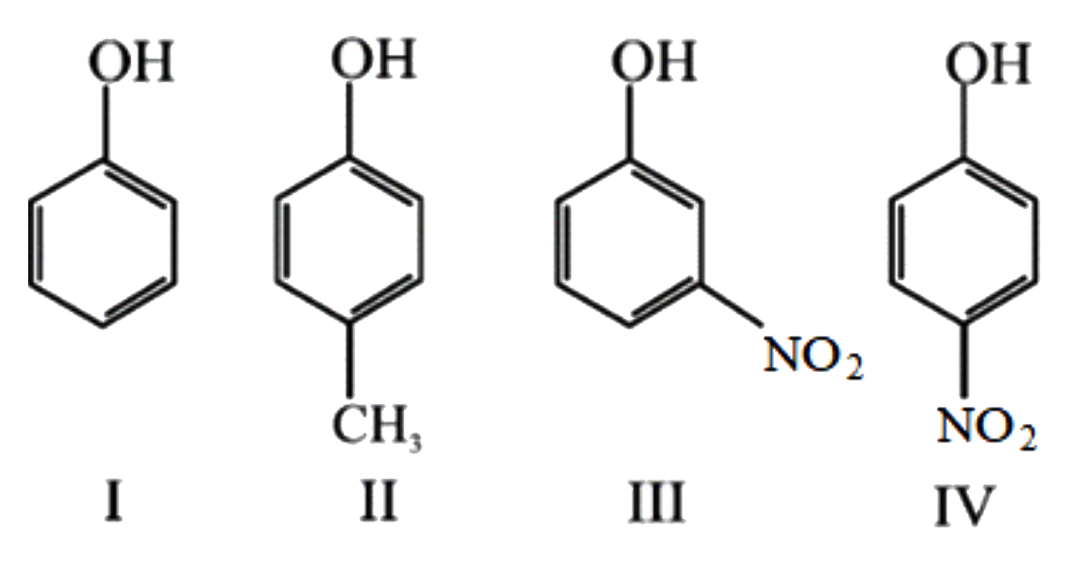
The order of acidity is
View Solution_E01_735_Q01.png) True or False
True or False
View SolutionWhich of the following is an achiral molecule?
View SolutionWhich of the following compund is achiral
View SolutionWhich of the following compound is achiral ?
View SolutionWhich of the following compound is achiral ?
View SolutionWhich of the following is an achiral molecule?
View SolutionWhich of the following molecule is achiral?
View SolutionWhich compounds is achiral in the following?
View SolutionWhich of the following amino acids are achiral ?
View SolutionWhich of the following molecules is achiral?
View SolutionWhich of the following molecules is achiral?
View SolutionWhich of the following is an achiral compound?
View SolutionWhich of the following molecules is achiral?
View SolutionWhich of the following is an achiral compound?
View SolutionWhich compounds is achiral in the following?
View Solution

