What happens when XeF6 reacts with NaF ?
To solve the question of what happens when xenon hexafluoride (XeF6) reacts with sodium fluoride (NaF), we can break down the reaction step by step.
Step 1: Identify the Reactants
The reactants in this reaction are:
- Xenon hexafluoride (XeF6)
- Sodium fluoride (NaF)
Step 2: Understand the Nature of the Reactants
Xenon hexafluoride is a compound that contains xenon and fluorine. Sodium fluoride is an ionic compound made up of sodium ions (Na⁺) and fluoride ions (F⁻).
Step 3: Predict the Reaction
When XeF6 reacts with NaF, it is expected to undergo a reaction where the fluoride ions from NaF can interact with XeF6. Since XeF6 can act as a Lewis acid, it can accept fluoride ions.
Step 4: Write the Reaction
The reaction can be represented as follows:
XeF6+NaF→Na++XeF−7
In this reaction:
- The sodium ion (Na⁺) is formed from the dissociation of sodium fluoride.
- The xenon heptafluoride ion (XeF7⁻) is formed as a result of the addition of an extra fluoride ion to XeF6.
Step 5: Conclusion
The final products of the reaction between xenon hexafluoride and sodium fluoride are sodium ions (Na⁺) and xenon heptafluoride ions (XeF7⁻).
Summary of the Reaction:
XeF6+NaF→Na++XeF−7
---
The reactants in this reaction are:
- Xenon hexafluoride (XeF6)
- Sodium fluoride (NaF)
Xenon hexafluoride is a compound that contains xenon and fluorine. Sodium fluoride is an ionic compound made up of sodium ions (Na⁺) and fluoride ions (F⁻).
When XeF6 reacts with NaF, it is expected to undergo a reaction where the fluoride ions from NaF can interact with XeF6. Since XeF6 can act as a Lewis acid, it can accept fluoride ions.
The reaction can be represented as follows:
XeF6+NaF→Na++XeF−7
- The sodium ion (Na⁺) is formed from the dissociation of sodium fluoride.
- The xenon heptafluoride ion (XeF7⁻) is formed as a result of the addition of an extra fluoride ion to XeF6.
The final products of the reaction between xenon hexafluoride and sodium fluoride are sodium ions (Na⁺) and xenon heptafluoride ions (XeF7⁻).
XeF6+NaF→Na++XeF−7
Topper's Solved these Questions
Similar Questions
Assertion (A): The enthalpy of formation of H2O(l) is greater than that of H2O(g).
Reason (R ) : Enthalpy change is negative for the condensation reaction H2O(g)→H2O(l).
View SolutionWhich of the following compound can show both optical and geometrical isomerism :
View SolutionWhat happenes when the follwing react with HNO2 ?
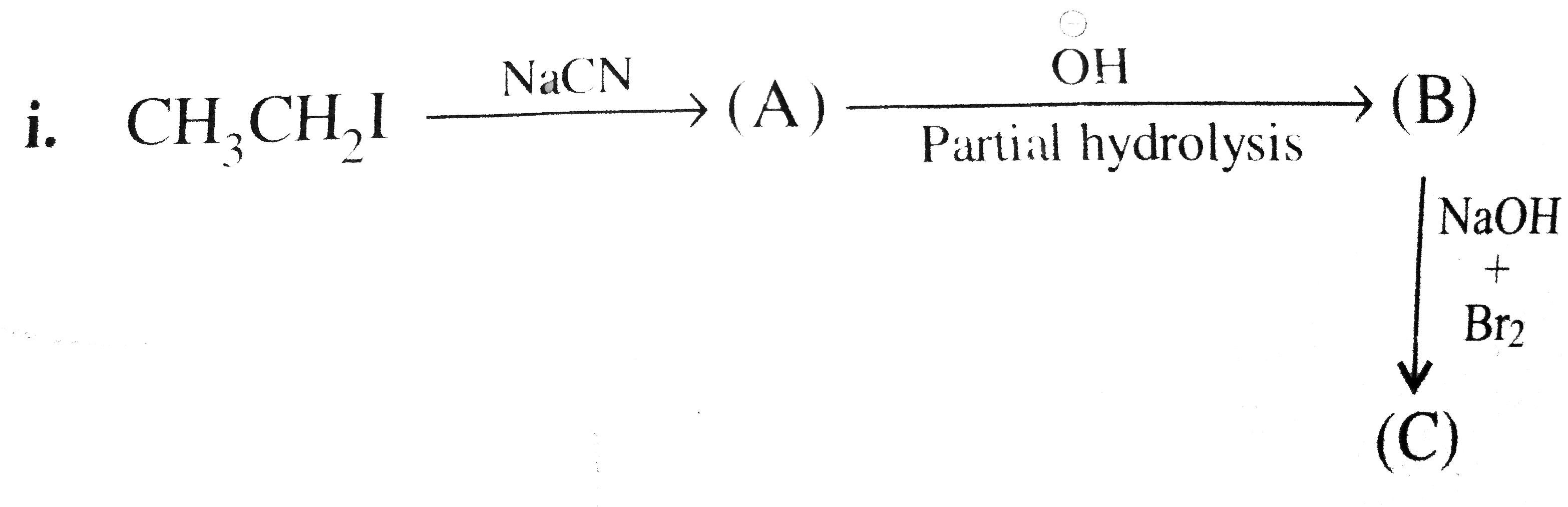 .
.
View SolutionWhat happens when :
Aluminium reacts with HNO3.
View SolutionWhen iodine is passed through aqueous solutions of NaF, NaBr and NaCl
View SolutionWhat happens when digydrogen reacts with dioxygen ?
View Solutionक्या होता है जब
(x)S8 कीCI2 के साथ अभिक्रिया कराई जाती है।
View SolutionBF_(3) reacts with NaH at 450 K to form NaF and X. When X reacts with LiH in diethyl ether, Y is formed. What is Y?
View SolutionWhat happens when XeF_(6) reacts 2.5 M solution of NaOH ?
View Solutionक्या होता है जब NaOH फेनॉल के साथ अभिक्रिया करता है?
View Solutionक्या होता है जब आयोडीन हाइपो विलयन के साथ क्रिया करती है ?
View Solutionक्या होता है जब शुष्क आयोडीन की ओजोन से अभिक्रिया होती है ?
View Solutionजब आयोडीन NaF, NaBr तथा NaCl रो क्रिया करता है, तो
View SolutionWhat happens when magnesium reacts with oxygen?
View SolutionWhen iodine reacts with NaF, NaBr and NaCl:
View SolutionWhat happens when formaldehyde reacts with ammonia?
View Solution
