(a) Account for the following :
(i) Acidic character increases from H2O to H2Te.
(ii) F2 is more reactive than ClF3 , whereas ClF3 is more reactive than Cl2.
(b) Draw the structure of (i) XeF2, (ii) XeOF4
.
(a) (i) Due to decrease in bond dissociation enthalpy of E-H
(ii) Due to lower bond dissociation enthalpy of F-F bond than Cl-F bond whereas Cl–Cl bond has higher bond dissociation enthalpy than Cl-F bond.
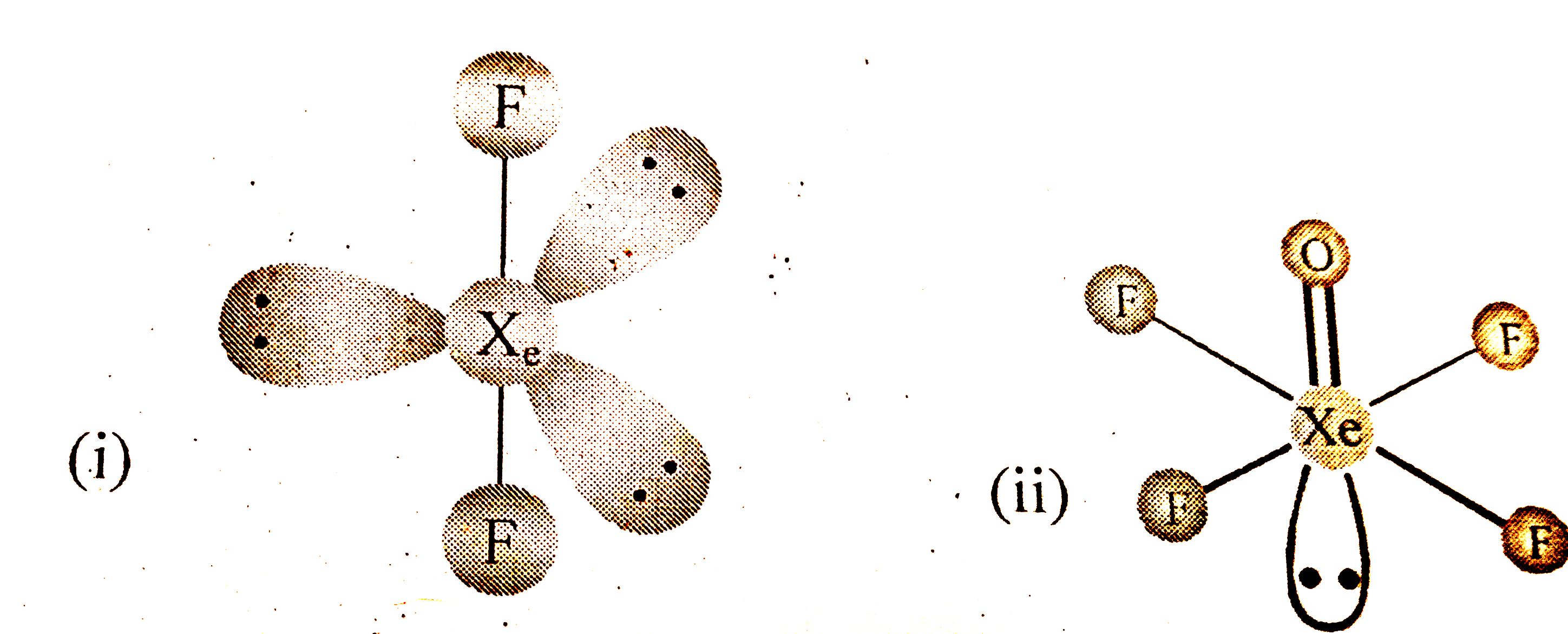
(c) 2F2(g)+2H2O(l)→4H+(aq)+4F–(aq)+O2(g)
Topper's Solved these Questions
Similar Questions
Assertion (A): The enthalpy of formation of H2O(l) is greater than that of H2O(g).
Reason (R ) : Enthalpy change is negative for the condensation reaction H2O(g)→H2O(l).
View SolutionWhich of the following compound can show both optical and geometrical isomerism :
View SolutionDraw the structure of the following molecules
(i) H3PO2(ii)H2S2O7(iii)XeOF4.
View SolutionComplete the following equations :
Ca3P2(s)+H2O(l)→
Cu2+(aq)+NH3(aq)→
F2(g)+H2O(l)→
Draw the structures of (i) H2S2O7(ii)BrF3 (iii)XeF2.
View Solution(a) (i) Why PCl5 gives fumes in moisture ?
(ii) Why Interhalogens are more reactive than pure halogens ?
(b) Draw the structures of the following :
(i)PCl5(ii)H2S2O8(iii)XeF4
View Solution(a) Write the balanced chemical equations for obtaining XeO3 and XeOF4from XeF6.
(b) Account for the followingk :
(i) H2S is less acidic then H2 Te.
(ii) H3PO2 has reducing nature.
(iii) SO2 is an air pollutant.
View Solution(a) Complete the following chemical equations :
(i) NaOH+Cl2→(hot and cone.)
(ii) XeF4+O2F2→
(b) Draw the structure of the following molecules :
(i) H3PO2
(ii) H2SO2O7
(ii) XeOF4
View Solutionनिम्नलिखित का बन्ध कोण लिखिए -
(i) CH4 , (ii) H2O , (iii) ClF3 , (iv) NH3 , (v) XeF2 |
View Solutionनिम्नलिखित अभिक्रियाओं को पूर्ण तथा सन्तुलित कीजिए-
(i) XeF2+H2O→
(ii) XeF4+H2O→
(iii) XeF6+H2O→
(iv) XeF6+NaF→
(v) XeOF4+H2O→
(vi) XeO3+H2O→
View SolutionGive reason for the following :
F2 is more reaction than ClF2 but ClF3 is more reactive than Cl2.
View SolutionWhy is H2 more reactive than D_(2) ?
View SolutionExplain why H2 is more reactive than D2.
View SolutionDraw the lewis structure of PCl5andSF6
View SolutionDraw Lewis dot structures for the following molecules : {:((a)C Cl_(4),(b)F_(2)O,(c )PF_(3),(d)F_(2)),((e )ClF_(3),(f)NCl_(3),(g)HONO,):}
View SolutionThe number of molecules having more than one lone pair among the following : XeF_(4), ClF_(3), NH_(3), SF_(4), XeF_(2), BrF_(5), H_(2)O
View SolutionGive reason for the following : F_(2) is more reactive than CIF_(3) but CIF_(3) is more reactive than Cl_(2) .
View Solution
