Which of the following statement is incorrect
AgCl<AgF solubility in H2O
KCl<KI solubility in acetone
BeC2O4<BaC2O4 solubility in H2O
CaCrO4>BaCrO4 solubility in H2O
The correct Answer is:C
(i) In AgCl more polarisation so less solubility
(ii) In KI more polarisation so more solubility in organic solvent
(iii) BeC2O4 more soluble due to high hydration energy
(iv) CaCrO4 has more solubility due to high hydration energy.
Topper's Solved these Questions
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
Explore 109 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)
Explore 2 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)
Explore 5 Videos
- HYDROGENBook:ALLENChapter:HYDROGENExercise:EXERCISE-5
Explore 17 Videos
- Ionic EquilibriumBook:ALLENChapter:Ionic EquilibriumExercise:All Questions
Explore 37 Videos
Similar Questions
The oxidation sate of molybdenum in its oxo complex species [Mo2O4(C2H4)2(H2O2)]2− is
View SolutionMatch the following columns

View Solution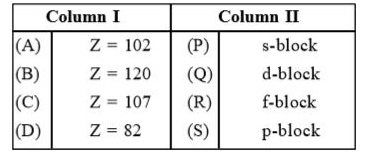
View SolutionAssertion : Cu react with HCl & form H_(2) gas
Reason : Cu lies above the hydrogen in electro chemical series
View SolutionThe percentage degree of hydrolysis of a salt of weak acid (HA) and weak base (BOH) in its 0.1 M solution is found to be 10%. If the molarity of the solution is 0.05 M, the percentage hydrolysis of the salt should be :
View SolutionWhich of the following statement is incorrect ? .
View SolutionWhich of the following statements is incorrect ?
View SolutionWhich of the following statement is incorrect ?
View SolutionWhich of the following statements is incorrect?
View SolutionWhich of the following statements are incorrect?
View SolutionIn which following statement is incorrect:
View SolutionWhich of the following statements is incorrect?
View SolutionWhich of the following statements is INCORRECT ?
View SolutionWhich of the following statements is incorrect?
View SolutionWhich of the following statement is incorrect :
View SolutionWhich of the following statements is an incorrect statement ?
View Solution
