PCI5 exists but NCI5 does not because
Nitrogen atom is much smaller than P
NCl5 is unstable
Nitrogen has no vacant d-orbitals
Nitrogen is highly inert
The correct Answer is:C
Step by step video solution for PCI_5 exists but NCI_5 does not because by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
Explore 109 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)
Explore 2 Videos
- INORGANIC CHEMISTRYBook:ALLENChapter:INORGANIC CHEMISTRYExercise:PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)
Explore 5 Videos
- HYDROGENBook:ALLENChapter:HYDROGENExercise:EXERCISE-5
Explore 17 Videos
- Ionic EquilibriumBook:ALLENChapter:Ionic EquilibriumExercise:All Questions
Explore 37 Videos
Similar Questions
The oxidation sate of molybdenum in its oxo complex species [Mo2O4(C2H4)2(H2O2)]2− is
View SolutionMatch the following columns

View Solution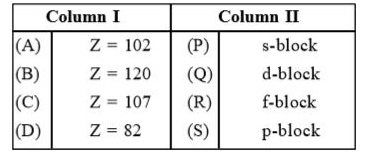
View SolutionAssertion : Cu react with HCl & form H_(2) gas
Reason : Cu lies above the hydrogen in electro chemical series
View SolutionThe percentage degree of hydrolysis of a salt of weak acid (HA) and weak base (BOH) in its 0.1 M solution is found to be 10%. If the molarity of the solution is 0.05 M, the percentage hydrolysis of the salt should be :
View SolutionPCl5 exists but NCl5 does not because :
View SolutionFeI_(3) does not exist because:
View SolutionSF_6 exists but OF_6 does not because
View SolutionWhich of the following does not react with PCI5
View SolutionIn case of nitrogen, NCl_(3) is possible but not NCI_5 gwhile in case of phosphorous, PCl_3 as well as PCI_5 IM are possible. It is due to
View SolutionPbI_(4) does not exist because:
View SolutionBCl_3 does not exist as dimer but BH_3 exist as dimer because
View SolutionBCI3 does not exist as dimer but BH, exist as dimer because
View SolutionPCl_5 exists but NCl_3 does not because:
View SolutionBCl_3 does not exist as dimer but BH_3 exist as dimer (B_2H_6) because :
View SolutionPbI4 does not exist because:
View Solution
