A certain sample of cuprous sulphide is found to have composition Cu1.8S, because of incorporation of Cu2+ ions in the lattice. What is the mole % of Cu2+ in total copper content in this crystal?Solution in Malayalam
0.998
0.1111
0.8888
0.3333
The correct Answer is:B
Two Cu+ replaced by one Cu2+, total loss of Cu=0.2 mole
So, mole of Cu2+ present in Cu1.8S=0.2∴% of Cu2+ in total copper content=0.21.8×100=11.11
Topper's Solved these Questions
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3
Explore 10 Videos
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3 (Multiple Correct Answer Type)
Explore 13 Videos
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-1
Explore 50 Videos
- REDOX REACTION & ELECTROCHEMISTRYBook:BRILLIANT PUBLICATIONChapter:REDOX REACTION & ELECTROCHEMISTRYExercise:QUESTION (ELECTROCHEMISTRY) (LEVEL -II) (ASSERTION-REASON)
Explore 3 Videos
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level-III (Linked Comprehension Type)
Explore 12 Videos
Similar Questions
The unit cell length of NaCl is observed to be 0.5627 nm by X-ray diffraction studies, the measured density of NaCl is 2.164 g cm−3. Calculate % of missing Na+andCl− ions.
View SolutionWhich of the following statements are correct ?
View SolutionThe radius of a di val ent cation M2+ is 94 pm and of divalent anion X2− is 146 pm. Thus, M2X2− has
View SolutionAssertion: After the electrolysis of aqueous CuSO4 solution pH decreases.
Reason: Sulphuric acid is formed.
View Solution1 mole of A and 1 mole B are mixed together to form an ideal solution at 25∘C. Solution is placed in a vessel fitted with piston. If pressure on the solution is reduced isothermally.
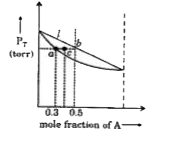
Correct relationship between P∘AandP∘B is
View SolutionA certain sample of cuprous sulphide is found to have composition Cu1.8S,, because of imcroporation of Cu2+ ion in the lattice, What is the mole % of Cu2+ in total content in this crystal?
View SolutionA certain sample of cuprous sulphide is found to have the composition Cu1.92S1.00 because of incorporation of Cu2+ and Cu+ ions in the crystal then ratio of Cu2+ and Cu+ ions is :
View SolutionCopper metal can be prepared by roasting copper ore, which contain cuprite (Cu_(2)S) and copper sulfide. (CuS) Cu_(2)S (s) + O_(2) (g) rarr 2Cu (s) + SO_(2) (g) CuS (s) + O_(2) (g) rarr Cu (s) + SO_(2) (g) If ore contains 14.6% impurity in addition to a mixture of CuS and Cu_(2)S . Heating 100 gm of mixture produces 70 gm of Cu metal with 90% purity. [Cu = 63] Percentage (By mass) of sulphur in the sample is :
View SolutionCopper metal can be prepared by roasting copper ore, which contain cuprite (Cu_(2)S) and copper sulfide. (CuS) Cu_(2)S (s) + O_(2) (g) rarr 2Cu (s) + SO_(2) (g) CuS (s) + O_(2) (g) rarr Cu (s) + SO_(2) (g) If ore contains 14.6% impurity in addition to a mixture of CuS and Cu_(2)S . Heating 100 gm of mixture produces 70 gm of Cu metal with 90% purity. [Cu = 63] Percentage (By mass ) of CuS in the sample is :
View Solutionक्यूप्रस क्लोराइड (Cu2Cl2) में Cu की संयोजकता ........... है।
View SolutionThe valency of Cu in cuprous chloride (Cu_(2)Cl_(2)) is ………….
View SolutionAssertion : Cuprous ion (Cu^(+)) has unpaired electrons while cupric ion (Cu^(++)) does not. Reason : Cuprous ion (Cu^(+)) is colourless where as cupric ion (Cu^(+2)) is blue in the aqueous solution.
View Solutionक्यूप्रस ऑक्साइड (Cu2O) तथा क्यूप्रिक ऑक्साइड (CuO) के एक मिश्रण का विश्लेषण करने पर उसमें 88% Cu पाया गया । मिश्रण का प्रतिशत संघटन ज्ञात करो । (दिया है : Cu=64,O=16)
View SolutionA certain sample of cuprous sulphide is found to have composition Cu1.8S, because of presence of some Cu2+ ions in the lattice. What is the mole % of Cu2+ in crystal ?
View SolutionCuprous sulphide and silver sulphide are isomorphous. The atomic mass of Cu is 63.57 g/mole. If the percentage composition of Sulphur in each of these is 20.14% and 12.94% respectively then the atomic mass of silver will be:
View Solutionप्रक्कथन : क्यूप्रस आयन (Cu+) में अयुग्मित इलेक्ट्रॉन होते हैं जबकि क्यूप्रिक आयन (Cu++) में नहीं होते।
कारण : क्यूप्रस आयन (Cu+) रंगहीन होते हैं जबकि क्यूप्रिक आयन (Cu++) जलीय विलयन में नीले होते हैं।
View Solution
