For the spinel structure (MgAl2O4), the correct statement is/areSolution in Malayalam
50% octahedral voids are occupied by Al3+ ions
Al3+ is equally distributed in tetrahedral voids and octahedral voids
Oxide ions occupy ccp lattice
12.5% tetrahedral voids are occupied by Mg2+ ions
The correct Answer is:A, C, D
For spinel structure, O2− ions form fcc arrangement.
∴Number of TV=8, Number of OV=4
Number of Mg2+ ions=18×TV=18×8=1
Number of Al3+ ions=12×OV=12×4=2.
So, 50% OVs are occupied and 1/8th i.e., 12.5% TVs are occupied.
Topper's Solved these Questions
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3 (Matching Column Type)
Explore 5 Videos
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3 (Numerical Type)
Explore 7 Videos
- SOLID STATEBook:BRILLIANT PUBLICATIONChapter:SOLID STATEExercise:LEVEL-3
Explore 10 Videos
- REDOX REACTION & ELECTROCHEMISTRYBook:BRILLIANT PUBLICATIONChapter:REDOX REACTION & ELECTROCHEMISTRYExercise:QUESTION (ELECTROCHEMISTRY) (LEVEL -II) (ASSERTION-REASON)
Explore 3 Videos
- SOLUTIONSBook:BRILLIANT PUBLICATIONChapter:SOLUTIONSExercise:Level-III (Linked Comprehension Type)
Explore 12 Videos
Similar Questions
Match the crystal systems/unit cells mentioned in Column I with their characteristic features mentioned in Column II.
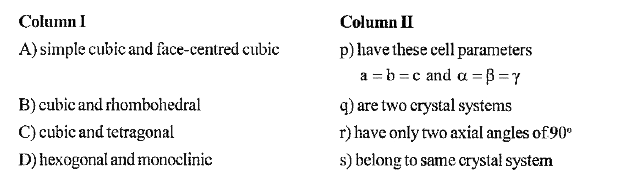
View SolutionDiamond structure is based on a face centred cubic lattice where four out of the eight tetrahedral holes are occupied by carbon atoms. Every atom in this structure is surrounded tetrahedrally by four other carbon atoms. How many carbon atoms per unit cell in diamond structure ?
View SolutionThe unit cell length of NaCl is observed to be 0.5627 nm by X-ray diffraction studies, the measured density of NaCl is 2.164 g cm−3. Calculate % of missing Na+andCl− ions.
View SolutionAssertion: After the electrolysis of aqueous CuSO4 solution pH decreases.
Reason: Sulphuric acid is formed.
View Solution1 mole of A and 1 mole B are mixed together to form an ideal solution at 25∘C. Solution is placed in a vessel fitted with piston. If pressure on the solution is reduced isothermally.
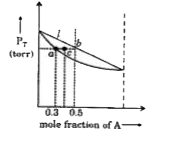
Correct relationship between P∘AandP∘B is
View SolutionFeIII(FeIIFeIII)O4 represent an inverse 2:3 spinel structre.
View SolutionWhat is the ratio of (TV/OV)occupied in spinel an inverse spinel structure?
View SolutionThe spinel structure (AB_(2)O_(4) consists of an fcc array of O^(2-) ions in which the:
View SolutionIn the spinel structur, oxides ions are cubical-closet packed whereas 1/8th of tetrahedral voids are occupied by A2+ cation and 1/2 of octahedral voids are occupied by B+ cations. The general formula of the compound having spinel structure is:
View SolutionWhich of the following represent a normal 2:3 spinel structure?
View SolutionWhich of the following compounds represents an inverse 2:3 spinel structure?
View SolutionWhich of the following compounds represents an inverse 2:3 spinel structure?
View SolutionThe CORRECT statements about the structure of H_(2)O_(2),O_(2)F_(2) and OF_(2) is /are
View SolutionAll spinel structures do not have
View SolutionIn a normal spinel structure , O^(2-) ions form fcc packing and 1/8 of the tetrahedral sites are occupied by divalent metal , A^(2+) ions and half of the octahedral sites are occupied by trivalent metal B^(3+) ions, what is the ratio of tetrahedral/octahedral sites : (i) occupied in spinel structure (ii) not occupied in spinel structure .
View SolutionThe formula of 'spinel' is AB_(2),O_(2) where Delta is......., and B is...
View Solution
