Which element has a full outermosť energy level containing only two electrons?
helium, He
fluorine, F
hydrogen, H
oxygen, O
The correct Answer is:A
helium, He
Topper's Solved these Questions
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:QUESTION BANK-20 ( Answer the following questions )
Explore 4 Videos
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:SELF ASSESSMENT SHEET -20
Explore 23 Videos
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:QUESTION BANK-20 (FILL IN THE BLANKS WITH APPROPRIATE WORDS TO MAKE CORRECT STATEMENTS. )
Explore 10 Videos
- CARBON AND ITS COMPOUNDS Book:S CHAND IIT JEE FOUNDATIONChapter:CARBON AND ITS COMPOUNDS Exercise:SELF ASSESSMENT SHEET-19
Explore 24 Videos
Similar Questions
What particle is needed to begin a nuclear chain reaction?
View SolutionWhat is the maximum number of electrons which can be accomodated in the L shell of an atom ?
View SolutionThe electrons present in the outermost shell of an atom are called_____ electrons.
View SolutionMatch correctly
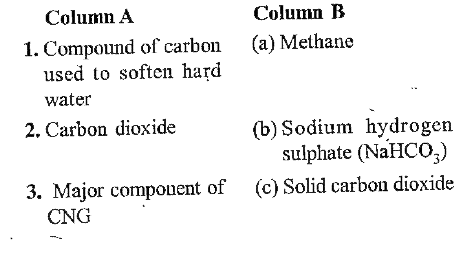
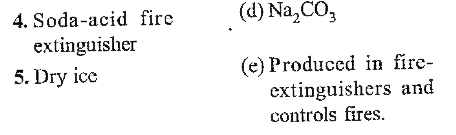
View SolutionAn element has atomic number 13 and an atomic mass of 27
(a) How many electrons are there in each atom of the element ?
(b) How are these electrons distributed in the various energy levels ?
View SolutionWhat is the maximum number of electrons present in the main energy level in which the 'g' subshell appears for the first time? Find the atomic number of the element to be discovered in which the differentiating electron is the only electron in the first 'g' subshell
View SolutionWhat is the maximum number of electrons present in the main energy level in which the g subshell appears for the first time? Find the atomic number of the element to be discovered in which the dif- ferentiating electron is the only electron in the first 'g' subshell.
View SolutionWhich element has only one electron in the d-orbitals ?
View SolutionName the element whose atom contains only one proton and one electrons.
View Solutionउस तत्व का इलेक्ट्रॉनिक विन्यास क्या होगा ? जिसके M-कोश में 5 इलेक्ट्रॉन है तथा K व L कोश पूर्ण भरे है ।
View SolutionAn orbital can contain only two electrons. Why?
View SolutionThe number of electrons in the highest principal energy level for an element(Z=26) is
View Solutionwhich period contains only two elements ?
View Solutionपोटेशियम परमाणु में इलेक्ट्रॉनिक ऊर्जा स्तरों का क्रम होता है
View SolutionHighest energy level of an electron corresponds to n=∞ and it has an energy of…….eV.
View Solutionपरमाणु संख्या 24 के तत्व के M ऊर्जा स्तर में इलेक्ट्रॉन की संख्या होगी:
View Solution