A carboxylic acide C2H4O2 reacts with an alcohol in the presence of H2SO4 to form a compound 'X'. The alcohol on oxidation with alkaline KMnO4 following by acidification gives the same caboxylic acid, C2H4O2. Write the name and structure of
Carboxylic acid.
To solve the problem step by step, we need to identify the carboxylic acid with the formula C2H4O2 and provide its name and structure.
Step 1: Identify the Carboxylic Acid
The molecular formula given is \( C_2H_4O_2 \). This indicates that the compound contains two carbon atoms, four hydrogen atoms, and two oxygen atoms.
Hint: Look for the general structure of carboxylic acids, which includes the carboxyl functional group (-COOH).
Step 2: Determine the Structure
A carboxylic acid has the functional group -COOH. Since we have two carbon atoms, we can represent the structure as follows:
1. One carbon will be part of the carboxyl group (-COOH).
2. The other carbon will be attached to the carboxyl group, making it an ethanoic acid.
The structure can be drawn as:
HO|∣|H−−C−−C−−OH∣H
This can also be written in a more condensed form as \( CH_3COOH \).
Hint: Remember that the carboxylic acid group consists of a carbonyl (C=O) and a hydroxyl (OH) group.
Step 3: Name the Carboxylic Acid
The name of the carboxylic acid with the formula \( C_2H_4O_2 \) is derived from the number of carbon atoms. Since there are two carbon atoms, it is called "ethanoic acid" (IUPAC name). It is also commonly known as "acetic acid."
Hint: The suffix "-oic acid" is used for naming carboxylic acids, while the prefix indicates the number of carbon atoms.
Final Answer
The carboxylic acid is Ethanoic Acid (IUPAC name) or Acetic Acid (common name), and its structure is:
HO|∣|H−−C−−C−−OH∣H
The molecular formula given is \( C_2H_4O_2 \). This indicates that the compound contains two carbon atoms, four hydrogen atoms, and two oxygen atoms.
A carboxylic acid has the functional group -COOH. Since we have two carbon atoms, we can represent the structure as follows:
2. The other carbon will be attached to the carboxyl group, making it an ethanoic acid.
HO|∣|H−−C−−C−−OH∣H
This can also be written in a more condensed form as \( CH_3COOH \).
The name of the carboxylic acid with the formula \( C_2H_4O_2 \) is derived from the number of carbon atoms. Since there are two carbon atoms, it is called "ethanoic acid" (IUPAC name). It is also commonly known as "acetic acid."
The carboxylic acid is Ethanoic Acid (IUPAC name) or Acetic Acid (common name), and its structure is:
Topper's Solved these Questions
- FOOTSTEPS TOWARDS CBSE BOARDBOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS CBSE BOARDEXERCISE - SECTION - C
39 Videos
- FOOTSTEPS TOWARDS CBSE BOARDBOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS CBSE BOARDEXERCISE - SECTION - C
39 Videos
- FOOTSTEPS TOWARDS (JEE MAIN) BOOK - MTG IIT JEE FOUNDATIONCHAPTER - FOOTSTEPS TOWARDS (JEE MAIN) EXERCISE - Integer/Numerical Value Type
5 Videos
- METALS AND NON METALS BOOK - MTG IIT JEE FOUNDATIONCHAPTER - METALS AND NON METALS EXERCISE - Olympiad/HOTS Corner
18 Videos
Similar Questions
The members of alkene family are represented by the general formula CnH2n. Now answer the following:
What do n and 2n signify?
View SolutionThe members of alkene family are represented by the general formula CnH2n. Now answer the following:
What do n and 2n signify?
View SolutionFrom the given list, how many pairs of elements form ionic bonds when they react?
Bromine, Carbon, Iron, Sodium, Sulphur
View SolutionMetals are extracted from their ores by different methods depending on their position in the activity series
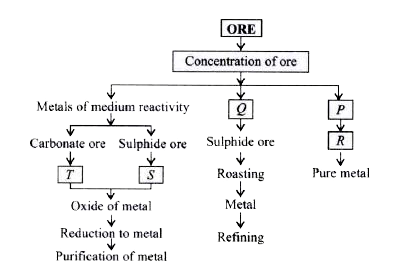
Match the Column-I with Column-II and select the correct option from the codes given below.
Column-I Column-II(A) Highly reactive metals(i) S (B) Calcination(ii) T (C) Electrolytic reduction(iii) P(D) Less reactive metal(iv) Q(E) Roasting(V) Q
View SolutionA compounds X is fromed by the reaction of a carboxylic acid C_(2)H_(4)O_(2) and an alcohol in the presence of a few drops of H_(2)SO_(4) . The alcohol on oxidation with alkalne KMnO_(4) followed by acidification gives the same carboxylic as used in this reaction. Give the names and structures of (a) carboxylic acid, (b) alcohol and (c ) the compound X. Also write the reaction.
View SolutionThe carboxylic acids react with hydrazoic acid in presence of H_(2)SO_(4) to form a primary amine. This reaction is called as :-
View SolutionA compound 'X' is formed by the reaction of carboxylic acid C2H2O4 and an alcohol in the presence of conc. H2SO4. This alcohol on treating with alkaline KMnO4 gives the sae carboxylic acid used in the reaction. Give the name structure of carboxylic acid and alcohol. Write the reaction involving formation of 'X'.
View SolutionA carboxylic acid C_(2)H_(4)O_(2) reacts with an alcohol in the presence of H_(2)SO_(4) to form a compound 'X'. The alcohol on oxidation with alkaline KMnO_(4) gives the same carboxylic acid, C_(2)H_(4)O_(2) . Write the name and structure of (i) Carboxylic acid, (ii) alcohol and (iii) the compound 'X'.
View SolutionA carboxylic acid is treated with an alcohol in presence of Conc. H2SO4. Name the reaction. Give its general equations.
View SolutionA carboxylic acid is heate with alcohol in presence of con. H_(2)SO_(4) . Name of the reaction Give its general equation.
View Solutionनिम्नलिखित सूत्रों में सम्भावित कार्योक्सिलिक अम्लों में यौगिकों के संरचनात्मक सूत्र लिखिए
(a)C4H8O2(b)C3H6O2
View SolutionA compounds X is fromed by the reaction of a carboxylic acid C2H4O2 and an alcohol in the presence of a few drops of H2SO4. The alcohol on oxidation with alkalne KMnO4 followed by acidification gives the same carboxylic as used in this reaction. Give the names and structures of (a) carboxylic acid, (b) alcohol and (c ) the compound X. Also write the reaction.
View SolutionA compound X is formed by the reaction of a carboxylic acid C_2H_4O_2 and an alcohol in the presence of a few drops of sulphuric acid. The alcohol on oxidation with alkaline potassium permanganate followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of the a) carboxylic acid b alcohol and c) the compound X. Also write the reaction.
View SolutionA compounds X is fromed by the reaction of a carboxylic acid C2H4O2 and an alcohol in the presence of a few drops of H2SO4. The alcohol on oxidation with alkalne KMnO4 followed by acidification gives the same carboxylic as used in this reaction. Give the names and structures of (a) carboxylic acid, (b) alcohol and (c ) the compound X. Also write the reaction.
View SolutionA carboxylic acid C2H4O2 reacts with an alcohol in the presence of H2SO4 to form a compound 'X'. The alcohol on oxidation with alkaline KMnO4 gives the same carboxylic acid, C2H4O2. Write the name and structure of (i) Carboxylic acid, (ii) alcohol and (iii) the compound 'X'.
View Solution
