Which of the following explain why propene undergo electrophilic addition with HBr, but not with HCN? Solution in Malayalam
Br− is better nucleophile than CN−
HBr being better source of proton as it is stronger acid than HCN
HCN attacks preferentially via lone pair of nitrogen
The C-Br bond being stronger is formed easily as compared to C-CN bond
The correct Answer is:B
HBr is a better source of proton. It gives H+ and Br− ions.
HBr→H++Br−
Thus, H+ attacks the π−bond of propene to form carbonium ion as
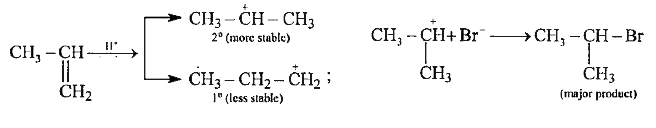
टॉपर्स ने हल किए ये सवाल
- ISOMERISM AND REACTION MECHANISMBook:BRILLIANT PUBLICATIONChapter:ISOMERISM AND REACTION MECHANISMExercise:LEVEL - III
Explore 8 Videos
- ISOMERISM AND REACTION MECHANISMBook:BRILLIANT PUBLICATIONChapter:ISOMERISM AND REACTION MECHANISMExercise:LEVEL - III (Multiple choice answer type)
Explore 15 Videos
- ISOMERISM AND REACTION MECHANISMBook:BRILLIANT PUBLICATIONChapter:ISOMERISM AND REACTION MECHANISMExercise:LEVEL - III (Linked Comprehension Type) (Paragraph III )
Explore 3 Videos
- HYDROGENBook:BRILLIANT PUBLICATIONChapter:HYDROGENExercise:LEVEL -III ( Linked Comprehension Type)
Explore 8 Videos
- ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART I (NOMENCLATURE) Book:BRILLIANT PUBLICATIONChapter:ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES - PART I (NOMENCLATURE) Exercise:LEVEL-III
Explore 45 Videos
समान प्रश्न
Equal amount of an RCl(C4H9Cl) is reacted at the same temperature with equal volume of 0.2 Mand 0.4M. solution of KOH, respectively, in two separate experiments. The time taken for the reaction of 50% of (C4H9Cl) was found to be same, the alkyl halide is:
View SolutionWhich of the following are S2N reactions?
View SolutionWhich of the following is most basic?
View SolutionThe volume of 10 volume H2O2, solution that decolourises 200 ml of 2NKMnO4, solution in acidic medium is:
View SolutionGive the IUPAC name of the following compound:
View SolutionWhy do alkenes prefer to undergo electrophilec addition reaction while arenes prefer electrophilic substitution reactions ? Explain.
View SolutionExplain why Aldehydes and ketones undergoes nucleophilic addition while alkenes undergoes electrophilic addition though both are unsaturated compounds.
View SolutionExplain the mechanism of addition of HBr to propene.
View SolutionExplain addition of HBr to Propene with the ionic mechanism
View SolutionExplain the mechanism of addition of HBr to propene
View SolutionThe addition of HBr to propene in the presence of peroxides follows ..........rule.
View SolutionWhy do alkenes undergo electrophilic addition reactions ?
View SolutionWhy do alkenes undergo electrophilic addition reactions ?
View SolutionWhy do alkenes undergo electrophilic addition reactions ? Explain the mechanism of HBr to ethylene.
View SolutionAddition of HBr to propene
View SolutionExplain the addition of HBr to propene by electrophilic addition mechanism and free radical addition mechanism.
View Solution
