The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume : SO2Cl2(g)→SO2(g)+Cl2(g)

Calculate the rate constant. (Given : log 4 = 0.6021, log 2 = 0.3010)Solution in Punjabi
Step by step video solution for The following data were obtained during the first order thermal decomposition of SO_2Cl_2 at a constant volume : SO_2Cl_2 (g) rarr SO_2(g)+ Cl_2(g) Calculate the rate constant. (Given : log 4 = 0.6021, log 2 = 0.3010) by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL KINETICSBook:MODERN PUBLICATIONChapter:CHEMICAL KINETICSExercise:EXERCISE
Explore 531 Videos
- BIOMOLECULESBook:MODERN PUBLICATIONChapter:BIOMOLECULESExercise:EXERCISE
Explore 503 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:MODERN PUBLICATIONChapter:CHEMISTRY IN EVERYDAY LIFEExercise:EXERCISE
Explore 352 Videos
Similar Questions
How can we express the rates of following reactions in terms of concentration of reactants and products ?
2O3↔3O2
View SolutionComplete the following statement- Uses for solder are-
View SolutionTrue or False : Equanil, used as tranquilizer, is a derivative of barbituric acid.
View SolutionFor I^(st) order decomposition of SO_2Cl_2(g) , SO_2Cl_2(g)toSO_2(g)+Cl_2(g) a graph of log (a-x) v//s t is shown in figure answer the following using above information. What is the rate constant (in sec^(-1) ) ?
View SolutionThe following data were obtained during the first order thermal decomposition of SO_(2) Cl_(2) at a constant volume : SO_(2)Cl_(2) (g) to SO_(2)(g)+Cl_(2)(g) {:("Experiment Times"//s^(-1) " Total pressure/atm"),("1 0 0.4" ),("2 100 0.7"):} Calculate the rate constant. Given : log 4 = 0.6021, log 2 = 0.3010)
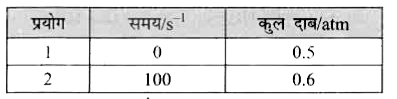
View Solutionस्थिर आयतन पर, SO2Cl2 के प्रथम कोटि के ताप अपघटन पर निम्न आकड़े प्राप्त हुए -
SO2Cl2(g)→SO2(g)+Cl2(g)
अभिक्रिया वेग की गणना कीजिए जब कुल दाब 0.65atm हो ।
Prayogsamay/skul daab/atm 100.521000.6
View SolutionFollowing data were obtained during first order thermal decomposition of - SO_(2)Cl_(2)(g)rarrSO_(2)(g)+Cl_(2)(g) {:("Experiment","time"(s^(-1)),"Total pressure"("atm")),(1,0,0.4),(2,100,0.7):} Calculate the rate constant . (Given : log4 = 0.6021, log2 = 0.3010)
View Solutionस्थिर आयतन पर SO2Cl2 के प्रथम कोटि के ताप अपघटन पर निम्न अकड़े प्राप्त हुए।
SO2Cl2(g)+Cl2(g)
View SolutionThe following data were given for thermal decomposition of SO_2Cl_2 at a constant volume : SO_2Cl_(2)(g) to SO_2(g) + Cl_(2) (g) Calculate the rate of the reaction when total pressure is 0.65 atm.
View SolutionThe following data were obtained during the first order thermal decomposition of SO_(2)Cl_(2) at a constant volume : SO_(2)Cl_(2)(g) rarr SO_(2)(g) + Cl_(2)(g) Calculate the rate constant. [Given : log 4 = 0.6021, log 2 = 0.3010]
View SolutionFor SO_2Cl_2(g)to SO_2(g) + Cl_2(g) Pressures of SO_2Cl_2 at t = 0 and t =20 minutes respectively are 700 mm & 350 mm. When log (P_0//p) is plotted against time (t), slope equals to
View Solutionकिसी निश्चित आयतन पर SO2Cl2 के प्रथम कोटि के तापीय विघटन के दौरान निम्न डाटा प्राप्त किया गया।
SO2Cl2(g)→SO2(g)+Cl2(g)
अभिक्रिया की दर क्या है जब कुल दाब 0.65 atm हो?
View SolutionThe following data were obtained for thermal decomposition of N_2O_5(g) at constant volume. 2N_2O_5(g) rarr 2N_2O_4(g)+O_2(g) Calculate the rate constant.
View SolutionThe following data were obtained during the first thermal decomposition of SO_2Cl_2 at a constant volume, SO_2Cl_2(g) to SO_2 (g)+Cl_2 (g) Experiment Time/s Total pressure/ atm 1 0 0.5 2 100 0.6 Calculate the rate of the reaction when total pressure is 0.65 atm.
View SolutionFor I^(st) order decomposition of SO_2Cl_2(g) , SO_2Cl_2(g)toSO_2(g)+Cl_2(g) a graph of log (a-x) v//s t is shown in figure answer the following using above information. What is the rate constant (in sec^(-1) ) ?
View SolutionThe following data were obtained during the first order thermal decomposition of N_2O_5 (g) at constant volume: 2N_2O_5 (g) rarr 2N_2O_4 (g) +O_2(g)
View Solution
