Which of the following is an incorrect set of quantum members?
n=2,l=0,m=0
n=1,/=0,m=0
n=3,l=3,m=0
n=2,l=1,m=1
The correct Answer is:c
To determine which set of quantum numbers is incorrect, we need to understand the rules governing quantum numbers. There are four quantum numbers:
1. Principal Quantum Number (n): This can take positive integer values (n = 1, 2, 3,...).
2. Azimuthal Quantum Number (l): This can take values from 0 to (n-1). For example, if n = 2, l can be 0 or 1.
3. Magnetic Quantum Number (m_l): This can take values from -l to +l. For example, if l = 1, m_l can be -1, 0, or +1.
4. Spin Quantum Number (m_s): This can take values of +1/2 or -1/2.
Now, let's analyze the possible sets of quantum numbers to identify the incorrect one.
1. Identify the Sets of Quantum Numbers: Review the given sets of quantum numbers. Each set should consist of four values: (n, l, m_l, m_s).
2. Check Each Set Against the Rules:
- For each set, check if the values of l, m_l, and m_s comply with the rules based on the value of n.
- Ensure that l is in the range of 0 to (n-1).
- Ensure that m_l is in the range of -l to +l.
- Ensure that m_s is either +1/2 or -1/2.
3. Example Analysis:
- Set 1: (n=2, l=1, m_l=0, m_s=+1/2)
- n = 2 → l can be 0 or 1 (valid)
- l = 1 → m_l can be -1, 0, +1 (valid)
- m_s = +1/2 (valid)
- Set 2: (n=3, l=2, m_l=1, m_s=-1/2)
- n = 3 → l can be 0, 1, or 2 (valid)
- l = 2 → m_l can be -2, -1, 0, +1, +2 (valid)
- m_s = -1/2 (valid)
- Set 3: (n=3, l=3, m_l=0, m_s=+1/2)
- n = 3 → l can be 0, 1, or 2 (invalid since l cannot equal n)
- Set 4: (n=2, l=1, m_l=1, m_s=-1/2)
- n = 2 → l can be 0 or 1 (valid)
- l = 1 → m_l can be -1, 0, +1 (valid)
- m_s = -1/2 (valid)
4. Conclusion: The incorrect set of quantum numbers is Set 3: (n=3, l=3, m_l=0, m_s=+1/2) because l cannot equal n.
Final Answer:
The incorrect set of quantum numbers is (n=3, l=3, m_l=0, m_s=+1/2).
2. Azimuthal Quantum Number (l): This can take values from 0 to (n-1). For example, if n = 2, l can be 0 or 1.
3. Magnetic Quantum Number (m_l): This can take values from -l to +l. For example, if l = 1, m_l can be -1, 0, or +1.
4. Spin Quantum Number (m_s): This can take values of +1/2 or -1/2.
- For each set, check if the values of l, m_l, and m_s comply with the rules based on the value of n.
- Ensure that l is in the range of 0 to (n-1).
- Ensure that m_l is in the range of -l to +l.
- Ensure that m_s is either +1/2 or -1/2.
- Set 1: (n=2, l=1, m_l=0, m_s=+1/2)
- n = 2 → l can be 0 or 1 (valid)
- l = 1 → m_l can be -1, 0, +1 (valid)
- m_s = +1/2 (valid)
- Set 2: (n=3, l=2, m_l=1, m_s=-1/2)
- n = 3 → l can be 0, 1, or 2 (valid)
- l = 2 → m_l can be -2, -1, 0, +1, +2 (valid)
- m_s = -1/2 (valid)
- Set 3: (n=3, l=3, m_l=0, m_s=+1/2)
- n = 3 → l can be 0, 1, or 2 (invalid since l cannot equal n)
- Set 4: (n=2, l=1, m_l=1, m_s=-1/2)
- n = 2 → l can be 0 or 1 (valid)
- l = 1 → m_l can be -1, 0, +1 (valid)
- m_s = -1/2 (valid)
The incorrect set of quantum numbers is (n=3, l=3, m_l=0, m_s=+1/2).
Topper's Solved these Questions
Similar Questions
The molarity of 720 g of pure water is
View SolutionIn a compound, atoms of elementsY form ccp lattice and those of element X occupy 2/3 rd of tetrahedral voids. The formula of the compound can be
View SolutionWhich of the following set of quantum numbers incorrect for last electron of fluorine atom ?
View SolutionSome of the following sets of quantum numbers are incorrect for d 4d electron. Which are the incorrect sets?
View SolutionAmong the following sets of quantum numbers, which one is incorrect for 4d-electrons-
View SolutionConsider the following sets of quantum number

Which of the following sets of quantum number is not possible ?
View SolutionConsider the following sets of quantum numbers which of the following sets of quantum number is not possible ?
View Solutionक्वाण्टम संख्याओं के निम्न सेटों पर विचार कीजिए
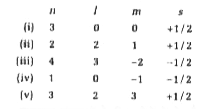
क्वाण्टम संख्याओं के निम्न सेटों में से कौन -सा संभव नहीं है ?
View SolutionConsider the following set of quantum numbers. Which of the following quantum numbers is not possible?
View SolutionWhich of the following sets of quantum numbers are followed ?
View Solutionक्वाण्टम संख्याओं के निम्नलिखित समुच्चयों में कौन-सा 4d-इलेक्ट्रॉन के लिए गलत है?
View SolutionThe set of quantum numbers members not applicable for an electron in an atom is
View SolutionConsider the following sets of quantum numbers

which of the following sets of quantum number is not possible ?
View Solutionક્વોન્ટમ આંક માટે નીચેના પૈકી કયું વિધાન ખોટું છે?
View SolutionThe incorrect of quantum numbers in the following is
View SolutionConsider the following sets of quantum number

Which of the following sets of quantum number is not possible?
View Solution
