For the reaction N2O4(g)→2NO2(g)
ΔH>ΔE
ΔH<ΔE
ΔH=ΔE
ΔH=0
The correct Answer is:a
To solve the question regarding the reaction N2O4(g)→2NO2(g), we need to analyze the relationship between the change in enthalpy (ΔH) and the change in internal energy (ΔE).
1. Identify the Reaction:
The reaction given is N2O4(g)→2NO2(g).
2. Determine the Change in Moles of Gas (ΔNG):
- The number of moles of gaseous products = 2 (from 2NO2).
- The number of moles of gaseous reactants = 1 (from N2O4).
- Therefore, ΔNG=moles of products−moles of reactants=2−1=1.
3. Use the Relationship Between ΔH and ΔE:
The relationship is given by the equation:
ΔH=ΔE+ΔNGRT
where R is the universal gas constant and T is the temperature in Kelvin.
4. Substitute ΔNG into the Equation:
We have ΔNG=1, so we can rewrite the equation as:
ΔH=ΔE+1⋅RT
or simply:
ΔH=ΔE+RT
5. Analyze the Relationship:
From the equation ΔH=ΔE+RT, it is clear that:
ΔH>ΔE
because RT is a positive term (as long as T is greater than 0).
6. Conclusion:
Therefore, the correct treatment for the reaction is that ΔH is greater than ΔE.
Final Answer:
The correct treatment for the reaction N2O4(g)→2NO2(g) is that ΔH>ΔE.
The reaction given is N2O4(g)→2NO2(g).
- The number of moles of gaseous products = 2 (from 2NO2).
- The number of moles of gaseous reactants = 1 (from N2O4).
- Therefore, ΔNG=moles of products−moles of reactants=2−1=1.
The relationship is given by the equation:
ΔH=ΔE+ΔNGRT
where R is the universal gas constant and T is the temperature in Kelvin.
We have ΔNG=1, so we can rewrite the equation as:
ΔH=ΔE+1⋅RT
or simply:
ΔH=ΔE+RT
From the equation ΔH=ΔE+RT, it is clear that:
ΔH>ΔE
because RT is a positive term (as long as T is greater than 0).
Therefore, the correct treatment for the reaction is that ΔH is greater than ΔE.
The correct treatment for the reaction N2O4(g)→2NO2(g) is that ΔH>ΔE.
टॉपर्स ने हल किए ये सवाल
समान प्रश्न
The molarity of 720 g of pure water is
View SolutionIn a compound, atoms of elementsY form ccp lattice and those of element X occupy 2/3 rd of tetrahedral voids. The formula of the compound can be
View SolutionGiven the following reaction : P : N_(2)(g)+2O_(2)(g)to2NO_(2)(g),DeltaH_(p)=16.18kcal Q : N_(2)(g)+2O_(2)(g)toN_(2)O_(4)(g),DeltaH_(Q)=2.31kcal Based on the above facts :
View SolutionAt the equilibrium of the reaction , N_(2)O_(4)(g)rArr2NO_(2)(g) , the observed molar mass of N_(2)O_(4) is 77.70 g . The percentage dissociation of N_(2)O_(4) is :-
View SolutionFor the first order reaction 2 N_(2)O_(5)(g) to 4 NO_(2) (g) + O_(2)(g)
View SolutionThe rate law for the reaction 2H_(2(g))+2NO_((g)) to N_(2(g))+2H_(2)O_((g)) is given by rate =-=k[H_(2)][NO]^(2) The reaction occurs in the following two steps: (a) H_(2(g))+2NO_((g)) to N_(2)O_((g))_H_(2)O_((g)) N_(2)O_((g))+ H_(2(g)) to N_(2(g))+H_(2)O_((g)) What is the rate of N_(2)O in the mechanism ? What is the molecularity of each of the elementary stpes ?
View SolutionWrite molecularity of the following reaction : 2NO_((s))+O_(2(g))to2NO_(2(g)) What is 'calcination' ? How does it differ from 'roasting' ? Write resonating structures of ozone, The decomposition of N_(2)O_(5(g)) at 320 K according to the following equation follows first order reaction : N_(2)O_(5(g))to2NO_(2(g))+(1)/(2)O_(2(g)) The initial concentration of N_(2)O_(5(g)) is 1*24xx10^(-2)mol.L^(-1) and after 60 minutes, 0*20xx10^(-2)mol.L^(-1) . Calculate the rate constant of the reaction at 320 K.
View SolutionThe molecularity and order of the reaction 2NO(g)+O_(2)(g)to2NO_(2)(g) are respectively
View SolutionIf in the reaction, N_(2)O_(4)(g)hArr2NO_(2)(g), alpha is the degree of dissociation of N_(2)O_(4) , then the number of moles at equilibrium will be
View Solutionअभिक्रिया 2N2O5(g)→2NO2(g)+O2(g) का अध्ययन किया गया एवं निम्नलिखित आँकड़े प्राप्त हुए
अभिक्रिया के लिये,
Q कोटि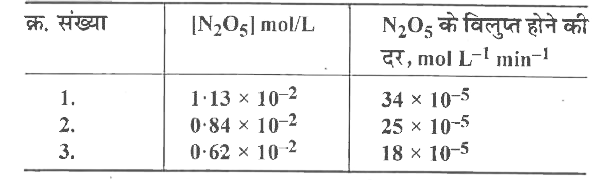
View SolutionAt the equilibrium of the reaction N_(2)O_(4) (g) rarr 2NO_(2)(g) the observed molar mass of N_(2) O_(4) is 77.7 gm . The percentage dissociation of N_(2)O_(4) is
View Solutionदी गई अभिक्रिया N2O4→2NO2+O2 के लिए Kp की इकाई है
View SolutionWhat is the expression for Keq for the reaction , 2N2O(g)+O2(g)⇔4NO(g)?
View SolutionFor the reaction 2NO(g)+O_(2)(g)to2NO_(2(g)) volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O_(2) and second order with respect to NO, the rate of reaction will
View SolutionFor the decomposition of N_(2)O_(5)(g) it is given that 2NOI_(2)O_(5(g))to4NO_(2(g))+O_(2(g)) .Activation energy E_(a), N_(2)O_(5(g))to2NO_(2(g))+1/2O_(2(g)) Activating energy E_(a)
View SolutionHow can you determine the rate law of the following reaction? 2NO_((g))+O_(2(g))to2NO_(2(g))
View Solution

