The pressure p and volume V of an ideal gas both increase in a process.Solution in Tamil
such a process is not possible.
The work done by the system is positive.
The tempreture of the system must increase.
Heat supplied to the gas is equal to the change in internal energy.
The correct Answer is:B, C
The work done by the system is positive., The tempreture of the system must increase.
Topper's Solved these Questions
- LAWS OF THERMODYNAMICSBook:HC VERMAChapter:LAWS OF THERMODYNAMICSExercise:Exercises
Explore 22 Videos
- LAWS OF THERMODYNAMICSBook:HC VERMAChapter:LAWS OF THERMODYNAMICSExercise:Short Answer
Explore 14 Videos
- LAWS OF THERMODYNAMICSBook:HC VERMAChapter:LAWS OF THERMODYNAMICSExercise:Objective I
Explore 9 Videos
- KINETIC THEORY OF GASESBook:HC VERMAChapter:KINETIC THEORY OF GASESExercise:All Questions
Explore 120 Videos
- NEWTON'S LAWS OF MOTIONBook:HC VERMAChapter:NEWTON'S LAWS OF MOTIONExercise:Exercises
Explore 42 Videos
Similar Questions
A thermally insulated, closed copper vessel contains water at 150C. When the vessel is shaken vigorously for 15 minutes, the temprature rises to 170C. The mass of the vessel is 100 g and that of the water is 200 g. the specific heat capacities of copper and water are 420Jkg−1K−1 and 4200Jkg−1K−1 respectively. negalect any thermal expansion.
(a) how much heat is transferred to the liquid-vessel system ?
(b)how much work has been done on this system?
(c ) how much is the increase in internal energy if the system ?
View SolutionShould the internal energy of a system necessarily increase if heat is added to it?
View SolutionThe first law of theromodynamics is a statement of
View SolutionCalculate the rms speed of nitrogen at STP (pressure =1 atm and temperature =00C. The density of nitrgoen in these conditions is 1.25kgm−3
View SolutionA block of mass 2 kg placed on a long frictionless horizontal table is pulled horizontally by a constant foerce F. It is found to move 10 m in the first two seconds. Find the magnitude of F.
View SolutionIn a isothermal process on an ideal gas, the pressure increases by 0.5% . The volume decreases by about.
View SolutionIn a process, the pressure of an ideal gas is proportional to square of the volume of the gas. If the temperature of the gas increases in this process, then work done by this gas
View SolutionAn ideal gas has initial volume V and pressure p. In doubling its volume the minimum work done will be in the process (of the given processes)
View SolutionThe pressure P and volume V of an ideal gas both decreases in a process.
View Solutionएक- परमाणुक आदर्श गैस के रुद्धोष्म प्रक्रम में दाब P तथा आयतन V में संबन्ध है:
View SolutionThe variation of pressure P with volume V for an ideal monatomic gas during an adiabatic process is hown in figure. At the magnitude of rate of change of pressure with volume is-
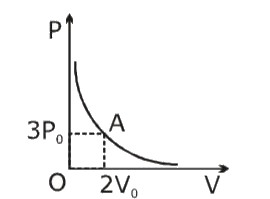
View SolutionThe variation of pressure P with volume V for an ideal monatomic gas during an adiabatic process is shown in figure . At point A the magnitude of rate of change of pressure with volume is
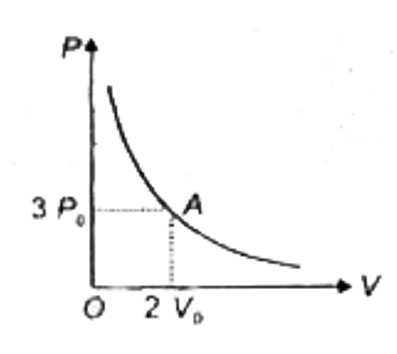
View SolutionAn ideal gas of volume V and pressure P expands isothermally to volume 16 V and then compressed adiabatically to volume V . The final pressure of gas is [γ=1.5]
View SolutionIn a isothermal process on an ideal gas, the pressure increases by 0.5% . The volume decreases by about.
View Solutionएक आदर्श गैस में दाब (P) के साथ आयतन (V) में रूद्धोष्म परिवर्तन होता है तब
View SolutionThe variation of pressure P with volume V for an ideal monoatomic gas during an adiabatic process is shown in figure. At point A the magnitude of rate of change of pressure with volume is
View Solution
