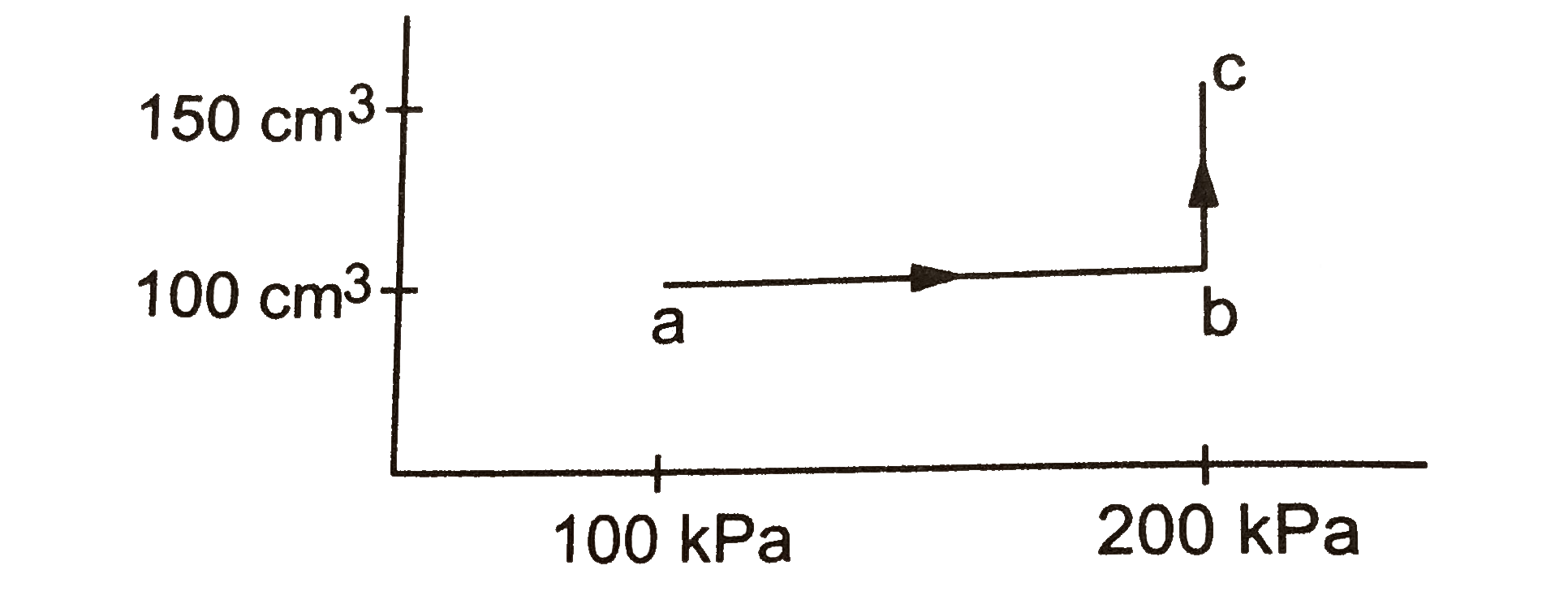
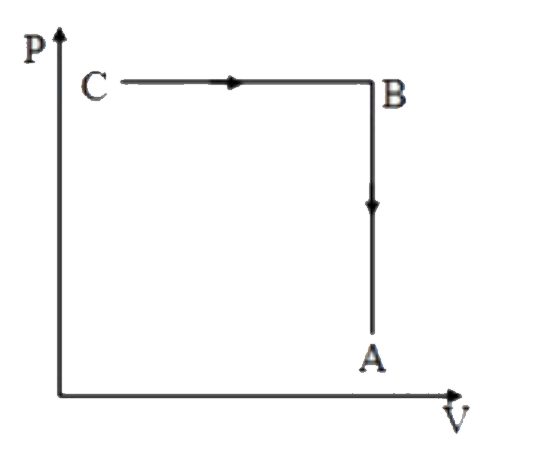
An ideal gas (γ=1.67) is taken through the process abc shown in figure . The temperature at the point a is 300k. Calculate (a) the temperature at b and c, (b) the work done in the process.
 Solution in Tamil
Solution in Tamil
(a) For a, b 'V' is constant
So, P1T1=P2T2
implies 100300=200T2
T2=(200×300100)=600K
For b, c 'p' is constant
So, V1T1=V2T2
implies 100600=150T2
implies T2(600×150100)=900K
(b) Work done = Area enclosed under the graph 50×20KPa
=50×10−6×200×103J
=10J.
(c) 'Q' supplied =nCvdT
Now,
Qbc=(PVRT)×(Rγ−1)×dT
=200×103×100×10−6×300600×0.67<br>=14.925( :. gamma = 1.67)<br>'Q'⊃plied→bc = nCpdT(C_p=(gammaR)/(gamma-1))<br>=(PV)/(RT) xx (gammaR)/(gamma-1) xxdT<br>=(200xx10^(3) xx 150 xx 10^(-6))/(600xx0.67)xx 300<br>=10xx(1.67)/(0.67)=24.925<br>(d)Q= DeltaU+W<br>Now,<br>DeltaU=Q-W<br>=Heat⊃plied−Workdo≠<br>=(24.925 + 14. 925)-10<br>=39.850-10=29.850J.
Topper's Solved these Questions
Similar Questions
A wave of wavelength 0.60 cm is produced in air and it travels at a speed of 300 ms^-1. Will it be audible?
View SolutionThe gravitational force acting on a particle of 1 g due to a similar particle is equal to 6.67×10−17N. Calculate the separation between the particles.
View SolutionAn ideal gas (γ=1.67) is taken through the process abc shown in figure . The temperature at the point a is 300k. Calculate (a) the temperature at b and c, (b) the work done in the process, ( c) the amount of heat supplied in the path ab and in the path bc and (d) the change in the internal energy of the gas in the process.

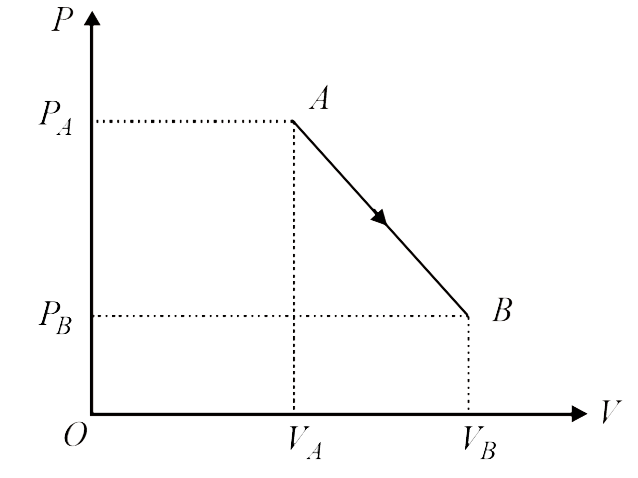
View SolutionAn ideal gas is taken from point A to the point B, as shown in the P-V diagram, keeping the temperature constant. The work done in the process is
View SolutionWork done by a sample of an ideal gas in a process A is double the work done in another process B. The temperature rises through the same amount in the two process. If CAandCB be the molar heat capacitites for the two processes:
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.
Net work done by the gas in the process is
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.

What is the greastes temperature of the gas during the cyclic process ?
View SolutionIdeal gas is taken through process shown in figure
View SolutionIdeal gas is taken through a process as shown in figure
View SolutionAn ideal gas is taken in a cyclic process as shown in the figure. Calculate (a) work done by the gas. (b) work done on the gas (c ) Net work done in the process
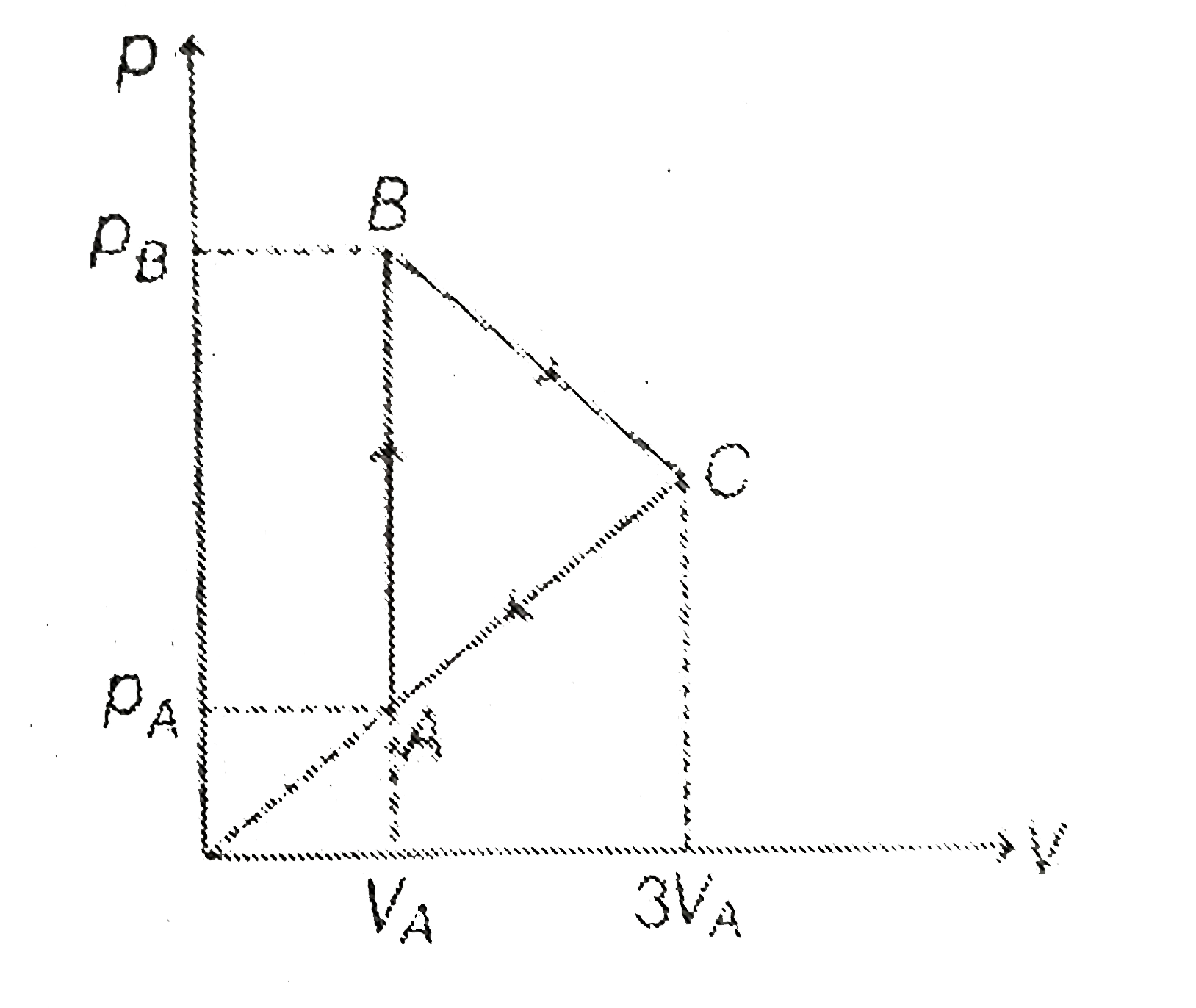
View Solutionn mol The ideal gas is shown in the figure AB Was taken through the process. What will be the maximum temperature of the gas in this process?
View SolutionAn ideal gas (gamma = 1.67 ) is taken through the process abc shown in figure . The temperature at the point a is 300 k . Calculate (a) the temperature at b and c, (b) the work done in the process, ( c) the amount of heat supplied in the path ab and in the path bc and (d) the change in the internal energy of the gas in the process.
View Solution300 K पर एक ग्राम मोल आदर्श गैस को रूद्धोष्म संपीड़ित करने पर अंतिम ताप 375 K हो जाता है। इस प्रक्रिया में किया गया कार्य लगभग होगा :- (R−8.3 जूल/मोल −K,γ=1.5)
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure. Calculate
The work done by the gas
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is T_(A) =200 K. In states B and C the temperature of the gas is the same. Net work done by the gas in the process is
View SolutionOne mole of monatomic gas is taken through cyclic process shown below. Temperature at A, T_(A)=300 K . The process A B is defined as P T= constant. If the work done in the process A B is (-k R) , then find k .(R= Universal gas constant) '(##CEN_KSR_PHY_JEE_C15_E01_017_Q10##)'
View Solution
