Bromine monochloride ( BrCl ) decomposes into bromine and chlorine according to reaction 2BrCl(g)=Br2(g)+Cl2(g),Kc=32 at 500 K. If initially, pure BrCl is taken at concentration 3.3×10−3mol L−1, what is its molar concentration in the mixture at equilibrium state?
1.23×10−2mol L−1
2.8×10−4M
3.54×10−3mol L−1
4.76×10−1mol L−1
The correct Answer is:B
2BrCl(g)⇔Br2(g)+Cl2(g),Kc
=32 at 500K
Initial concentration3.30×10−3mol L−100Equilibrium concentration(3.30×10−3−x)mol L−1x2mol L−1x2mol L−1
Kc=[Br2][Cl2][BrCl]2=x/2×x/2(3.30×10−3−x)2=32
x24×(3.30×10−3−x)2=32
x2×(3.30×10−3−x)=√32=5.656
x3.30×10−3−x=2×5.656=11.312
x=11.31(3.30×10−3−x)
x=0.3732−11.312x
x+11.312x=0.037372 i.e., x=3.02×10−3
[BrCl]equilibrium=(3.30−3.02)×10−3
=0.28×10−3
=2.8×10−4M
Topper's Solved these Questions
Similar Questions
Eutrophication causes reduction in
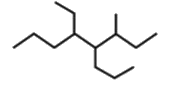
View SolutionThe correct IUPAC name of the following hydrocarbon is
View SolutionBromine monochloride, (BrCl) decomposes into bromine and chlorine and reaches the equilibrium. 2BrCl_((g))hArrBr_(2(g))+Cl_(2(g)) For which K_(c)=32 at 500 K . If initially pure BrCl is present at a concentration of 3.30xx10^(-3) mol litre^(-1) , what is its molar concentration in the mixture at equilibrium?
View SolutionCalculate the free eneryg change at 298 K for the reaction , Br_(2)(l)+Cl_(2)(g) to BrCl(g) . For the reaction DeltaH^(@)=29.3 kJ &
View SolutionCalculate the free energy change at 298 K for the reaction, Br_(2)(l)+ Cl_(2)(g) rarr 2BrCl(g) . For the reaction DeltaH^(@)=29.3 KJ & the entropies of Br_(2)(l),Cl_(2)(g) & BrCl(g) at the 298 K are 152.3,223.0,239.7 J "mol^(-1)K^(-1) respectively.
View SolutionBromine monochloride (BrCl) (g) hArr Br_(2) (g) + Cl_(2) (g) for which K_(c) = 32 " at " 500 K . If initially pure BrCl is present at a concentration of 3*30 xx 10^(-3) " mol " L^(-1) , what is its molar concentration in the mixture at equilibrium ?
View Solutionब्रोमीन मोनोऑक्सीडे BrCl विघटित होकर ब्रोमीन एवं कलरोइने देता है तथा साम्य स्थापित होता है-
2BrCl(g)<⇒Br2(g)+Cl2(g)
इसके लिए 500 K पर KC=32 है। यदि प्रारम्भ में BrCl की सांद्रता 3.3×10−3molL−1 हो, तो साम्य पर मिश्रण में इसकी सांद्रता क्या होंगी?
View SolutionBromine monochloride, BrCl decomposes into bromine and chlorine and reaches the equilibrium: 500K. If initially pure BrCl is present at a concentration of 3.3xx10^(-3)mol*L^(-1) , what is its molar concentration in the mixture at equilibrium?
View SolutionWhich of the following relations regarding the reaction : Br_(2) (l) + Cl_(2) (g) to 2 BrCl(g) is correct -
View SolutionCalculate the free energy change at 298 K for the reaction , Br2(l)+Cl2(g)→2BrCl(g). For the reaction ΔH∘=29.3kJ & the entropies of Br2(l),Cl2(g)&BrCl(g) at the 298 K are 152.3, 223.0, 239.7 Jmol−1K−1 respectively.
View SolutionFor the reaction 2BrCl hArr Br_(2)(g) + Cl_(2)(g) K_(c)" is " 32 " at " 500 K . If initially pure BrCl is present at a concentration of 3.30 xx 10^(-3) , M, what is its molar concentration in the mixture at equilibrium?
View SolutionThe bond between chlorine and bromine in BrCl is
View SolutionBromine monochloride, BrCl decomposes into bromine and chlorine and reaches the equilibrium :
2BrCl(g)⇔Br2(g)+Cl2(g)
for which Kc=32 at 500K. If initially pure BrCl is present at a concentration of 3.3×10−3molL−1, what is its molar concentration in the mixture at equilibrium?
View SolutionBromine monochloride, (BrCl) decomposes into bromine and chlorine and reaches the equilibrium. 2BrCl_((g))hArrBr_(2(g))+Cl_(2(g)) For which K_(c)=32 at 500 K . If initially pure BrCl is present at a concentration of 3.30xx10^(-3) mol litre^(-1) , what is its molar concentration in the mixture at equilibrium?
View SolutionThe bond between chlorine and bromine in BrCl is
View SolutionBromine monochloride, BrCl decomposes into bromine and chlorine and reaches the equilibrium :
2BrCl(g)⇔Br2(g)+Cl2(g)
for which Kc=32 at 500K. If initially pure BrCl is present at a concentration of 3.3×10−3molL−1, what is its molar concentration in the mixture at equilibrium?
View Solution

