The decreasing order of acidity of following phenol derivatives is
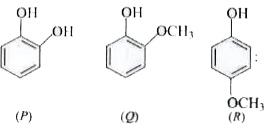
A
P>Q>R
B
R>Q>P
C
Q>P>R
D
P>R>Q
Text Solution
The correct Answer is:D
−Mand−I will increase acidity -M effective at ortho and para positions only while -I effect will operate at all position but it is distance dependent.
+M and +I will decrease acidity but +M will be effective at ortho and para only.
H-bonding in phenol derivative is also consider for the comparison of acidity.
|
Similar Questions
Most acidic phenol derivative is
View SolutionArrange the following in decreasing order of acidity Phenol, o-nitrophenol, o-cresol
View SolutionConsider the following phenols : The decreasing order of acidity of the above phenols is
View SolutionArrange p-nitrophenol, o-nitrophenol and phenol in order of decrease in acidity.
View SolutionThe decreasing order of acidity of following benzoic acid derivatives is
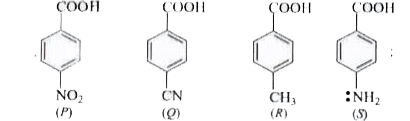
View SolutionThe decreasing order of acidity of following benzoic acid derivatives is
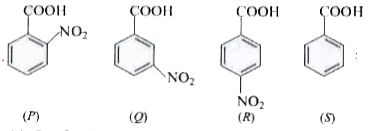
View SolutionThe decreasing order of acidity of following benzoic acid derivatives is
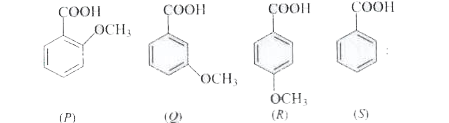
View SolutionThe decreasing order of acidity of following benzoic acid derivatives is
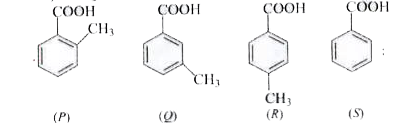
View Solution

