In an aromatic ring, a functional group with lone pair of electron exerts +M effect, some functional groups like, -NO, -NC, −CH=CH2 etc., can function both as electron donating (+M) or electron withdrawing (-M) groups. More extended conjugation provide more stabilization.
The most stable resonating structure is :
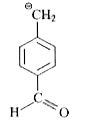
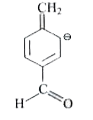
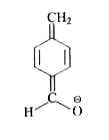
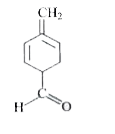
The correct Answer is:C
−ive charge present on electronegative oxygen.
Similar Questions
The concept of resonance explains various properties of compounds.The molecules with conjugated system of π bonds, are stabilized by resonace and have low heat of hydrogenation.Hyperconjugative stabilization also decreases heat f hydrogenation.In aromic rings a functional group with a lone pair of electron exerts +m effect. Some functional groups like -NO, -NC, -CH=CH2 can function both as electron releasing (+m,+R) or electron withdrawing (-m,-R) groups.More extended conjugation provides more stabilization.
The most stable carbocation is :
View SolutionIdentify the functional group that has electron donating inductive effect.
View SolutionExplain the effect of electron withdrawing groups. [EWG] on the acidity of carboxylic acids. Give examples.
View SolutionMesomerism is extended resonance. Atoms or groups with lone pairs of electrons release electrons when connected to doubly bonded carbons. It is +M effect. Unsaturated groups when connected to doubly bonded carbons withdraw π electrons from the doubly bonded carbons. It is -M effect. Here the double bond is the unsaturated group should be between less electronegative atom and more electronegative atom.
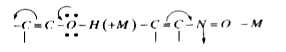
Mesomeric effect stabilizes or destabilizes the conjugate base of an acid. As a result the acid may be strong or weak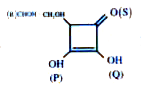 has 4(OH) groups. Which -OH is a strong acid?
has 4(OH) groups. Which -OH is a strong acid?
View SolutionMesomerism is extended resonance. Atoms or groups with lone pairs of electrons release electrons when connected to doubly bonded carbons. It is +M effect. Unsaturated groups when connected to doubly bonded carbons withdraw π electrons from the doubly bonded carbons. It is -M effect. Here the double bond is the unsaturated group should be between less electronegative atom and more electronegative atom.
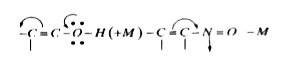
Which of the following is most stable?
View SolutionMesomerism is extended resonance. Atoms or groups with lone pairs of electrons release electrons when connected to doubly bonded carbons. It is +M effect. Unsaturated groups when connected to doubly bonded carbons withdraw π electrons from the doubly bonded carbons. It is -M effect. Here the double bond is the unsaturated group should be between less electronegative atom and more electronegative atom.
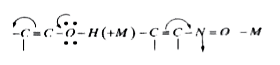
Which of the following statements is right?
View SolutionWhich of the following groups can neither donate a pair of electrons in resonance
View SolutionIdentify the functional group that has electron donating inductive effect.
View Solution

