Can the following groups of elements be classified as Dobereiner's triad:
(a) Na, Si, Cl (b) Be, Mg, Ca
Atomic mass of Be−9,Na−23,Mg−24,Si−28,Cl−35,Ca−40. Justify your answer in
each case.
Na, Si, Cl - The properties of these three elements are not similar to each other, so no Dobereiners triad.
Be, Mg, Ca - The properties are similar to each other so it is Dobereiner.s triad.
Atomic mass of Mg = Atomic mass of Be + Atomic mass of Ca2
=24.5
Topper's Solved these Questions
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (LONG ANSWER)
30 Videos
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (MULTIPLE CHOICE QUESTIONS)
13 Videos
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (VERY SHORT ANSWER)
9 Videos
- CHEMICAL REACTIONS AND EQUATIONSBOOK - OSWAL PUBLICATIONCHAPTER - CHEMICAL REACTIONS AND EQUATIONSEXERCISE - CASE-BASED MCQs
29 Videos
- METALS AND NON - METALS BOOK - OSWAL PUBLICATIONCHAPTER - METALS AND NON - METALS EXERCISE - CASE - BASED MCQs
29 Videos
Similar Questions
Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
View SolutionThe electronic configuration of an element M is 2,8,4. In modern periodic table, the element M is placed in
View SolutionWhich element was named as Eka-aluminium earlier?
View SolutionMarble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is
View SolutionRead the following ans answer any four metals A,B,C and D added following solutions one. The results obtained have tabulated as follows :
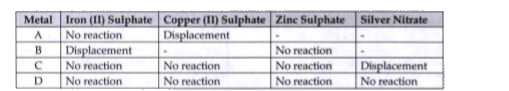
Choose the most reactive metal:
View SolutionArrange the following elements in the order of their decreasing metallic charcter Na, Si, Cl, Mg, Al.
View SolutionCan the following groups of elements be classified as Dobereiner's triad ? (a) Na, Si, Cl (b) Be, Mg, Ca Atomic mass of Be 9 , Na 23 , Mg 24 , Si 28 , Cl 35 , Ca 40 Explain by giving reason.
View SolutionCan the following groups of elements be classified as Dobereiner's triad:
(a) Na, Si, Cl (b) Be, Mg, Ca
Atomic mass of Be−9,Na−23,Mg−24,Si−28,Cl−35,Ca−40. Justify your answer in
each case.
View Solution3rd period : Na - Mg -Al - SI -P -S -Cl . Classify them into metals , non -metals and metalloids .
View SolutionCan the following groups of elements be classified as Dobereiner's triad? (a)Na, Si, ci (b) Be, Mg, Ca Atomic mass of Be 9, Na 23, Mg 24, Si 28, Cl 35, Ca 40 Explain by giving reason.
View SolutionClassify the following elements into groups of triads :
Cl, Ca, Ba, Sr, K, Li, Br, I, Na
View SolutionCan the following group of elements be classified as Dobereiner's Triad ?
Na,Si,CI
View SolutionArrange the following elements in the order of their decreasing metallic character Na, Si, Cl, Mg, Al.
View SolutionCan the following groups of elements be classified as Dobereiner's Triads.
i] Na, Si, Cl ii] Be, Mg, Ca<br>A→micmassofBe - 9. Na - 23, Mg -24, Si - 28, CI - 35, Ca - 40. Justify your answer.
View SolutionArrange the following elements in the order of their decreasing metallic charcter Na, Si, Cl, Mg, Al.
View SolutionCan the following groups of elements be classified as Dobereiner's triad ?
(a) Na, Si, Cl
(b) Be, Mg, Ca
Atomic mass of Be 9 , Na 23 , Mg 24 , Si 28 , Cl 35 , Ca 40
Explain by giving reason.
View Solution
