Arrange the following elements in the increasing order of their:
(i) atomic radii
(ii) non metallic character. Give reason for your
answer.
Li, Be, F, N
To arrange the elements Li, Be, F, and N in increasing order of their atomic radii and non-metallic character, we need to understand the trends in the periodic table.
Step 1: Arrange the elements in increasing order of atomic radii
1. Understanding Atomic Radii: Atomic radius is the distance from the nucleus to the outermost shell of an electron. Atomic radii generally decrease across a period from left to right due to the increasing nuclear charge, which pulls the electrons closer to the nucleus. Conversely, atomic radii increase down a group due to the addition of electron shells.
2. Elements in Question: The elements are:
- Lithium (Li) - Group 1, Period 2
- Beryllium (Be) - Group 2, Period 2
- Nitrogen (N) - Group 15, Period 2
- Fluorine (F) - Group 17, Period 2
3. Comparison:
- Li has the largest atomic radius because it is the furthest left in the period.
- Be is smaller than Li but larger than N and F.
- N is larger than F because N is to the left of F in the same period.
- F has the smallest atomic radius due to the highest nuclear charge in this group.
4. Increasing Order of Atomic Radii:
- F < N < Be < Li
Step 2: Arrange the elements in increasing order of non-metallic character
1. Understanding Non-metallic Character: Non-metallic character refers to the tendency of an element to gain electrons and form negative ions. Non-metallic character increases across a period from left to right and decreases down a group.
2. Comparison:
- Li is a metal and has the least non-metallic character.
- Be is also a metal but has slightly more non-metallic character than Li.
- N is a non-metal and has a higher non-metallic character than both Li and Be.
- F is a highly electronegative non-metal and has the highest non-metallic character among these elements.
3. Increasing Order of Non-metallic Character:
- Li < Be < N < F
Final Answers:
- Increasing order of atomic radii: F < N < Be < Li
- Increasing order of non-metallic character: Li < Be < N < F
- Lithium (Li) - Group 1, Period 2
- Beryllium (Be) - Group 2, Period 2
- Nitrogen (N) - Group 15, Period 2
- Fluorine (F) - Group 17, Period 2
- Li has the largest atomic radius because it is the furthest left in the period.
- Be is smaller than Li but larger than N and F.
- N is larger than F because N is to the left of F in the same period.
- F has the smallest atomic radius due to the highest nuclear charge in this group.
- F < N < Be < Li
- Li is a metal and has the least non-metallic character.
- Be is also a metal but has slightly more non-metallic character than Li.
- N is a non-metal and has a higher non-metallic character than both Li and Be.
- F is a highly electronegative non-metal and has the highest non-metallic character among these elements.
- Li < Be < N < F
- Increasing order of atomic radii: F < N < Be < Li
- Increasing order of non-metallic character: Li < Be < N < F
Topper's Solved these Questions
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (LONG ANSWER)
30 Videos
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (MULTIPLE CHOICE QUESTIONS)
13 Videos
- DIKSHA QUESTIONS BOOK - OSWAL PUBLICATIONCHAPTER - DIKSHA QUESTIONS EXERCISE - PERIODIC CLASSIFICATION OF ELEMENTS (VERY SHORT ANSWER)
9 Videos
- CHEMICAL REACTIONS AND EQUATIONSBOOK - OSWAL PUBLICATIONCHAPTER - CHEMICAL REACTIONS AND EQUATIONSEXERCISE - CASE-BASED MCQs
29 Videos
- METALS AND NON - METALS BOOK - OSWAL PUBLICATIONCHAPTER - METALS AND NON - METALS EXERCISE - CASE - BASED MCQs
29 Videos
Similar Questions
Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
View SolutionThe electronic configuration of an element M is 2,8,4. In modern periodic table, the element M is placed in
View SolutionWhich element was named as Eka-aluminium earlier?
View SolutionMarble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is
View SolutionRead the following ans answer any four metals A,B,C and D added following solutions one. The results obtained have tabulated as follows :
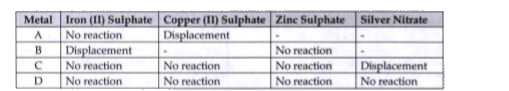
Choose the most reactive metal:
View SolutionArrange the following elements in the order of their increasing non-metallic character Li, O, C, Be, F
View SolutionArrangement the following elements in increasing order of their atomic radii
(a) Li, Be, F, N
(b) Cl, At, Br, I
View SolutionArrange the elements N, P, O and S in the order of i) increasing first ionisation enthalpy. ii) increasing non-metallic character. Give reason for the arrangement assigned.
View SolutionArrange the following element in the increasing order of their non - metals character : B , C, Si N , F
View SolutionArrange the following elements in the increasing order of non-metallic character: B, C, Si, N, F.
View SolutionArrange the following elements in the increasing order of non - metallic character. B,C,Si,N,F
View SolutionArrange the following elements in increasing order of non - metallic character : B, C, Si , N, F .
View SolutionWrite the correct increasing order of non-metallic character for B,C,N,F and Si.
View SolutionArrange the following elements in the increasing order of non- metallic character: B, C, Si, N and F.
View SolutionArrangement the following elements in increasing order of their atomic radii
(a) Li, Be, F, N
(b) Cl, At, Br, I
View SolutionArrange the following elements in the order of their increasing non-metallic character
Li,O,C,Be,F
View Solution
