Read the following text and answer the following questions on the basis of the same:
Atomic Absorption Spectrometer: The atomic absorption (AA) spectrometer is used to analyze metals at very low concentrations, typically in the parts per million (ppm) or parts per billion (ppb) ranges. A liquid sample containing dissolved material whose concentration is to be measured is aspirated into a thin, wide AA flame, or is introduced into a small carbon furnace which is heated to a high temperature.
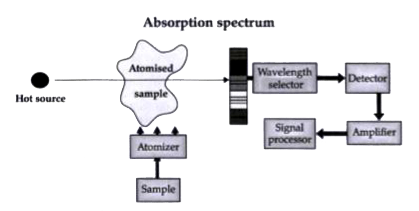
Basic Principle of AAS is the measurement of absorption of radiation by free atoms. The total amount of absorption depends on the number of free atoms present and the degreee to which the free atoms absorb the radiation. At the high temperature of the AA flame, the sample is broken down into atoms using an atomizer and it is the concentration of these atoms that is measured. Sample in the form of solution is used. It is broken up into a fine mist with the help of an atomizer. When the mist reaches the flame, the intense heat breaks up the sample into its individual atoms. When a photon coming out from the hot source hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, then the electron in the lower energy level absorb the photon and jumps up to the higher energy level. If the photon energy does not correspond to the difference between two energy levels, then the photon will not be absorbed (it may be scattered away). Hence in the spectrum, the wavelength corresponding to the absorbed photons is observed as black lines as shown in the following spectrum of Hydrogen. The dark lines correspon to the frequencies of light those have been absorbed by the sample element. Using this process, a source of photons (generally a white light) of various energies is used to obtain the absorption spectra of different materials and to identify them.
What is the basic principle of Atomic Absorption Spectrophotometer?
Emission of photons when excited electron of an atom comes back to lower energy level.
Absorption of photons when electrons at lower energy level jumps to a higher energy level.
Emission of electrons from an atom at a very high temperature.
Emission of electron when energetic photons bombard an atom.
The correct Answer is:B
Basic principle of AAS is the measuremetn of absorption of radiation by free atoms. When a photon hits an atom and the energy of the photon is equal to the gap between two electron energy levels of the atom, the electron in the lower energy level absorb the photon and jumps up to the higher energy level.
Similar Questions
Mole Fraction|Questions|Parts Per Million (PPM) / Parts Per Billion (PPB)|Questions|Summary
View Solutionप्रति हजार के भाग में समुद्र की नमक सांद्रता, जो मापी गई है
View SolutionDefine the terms : Parts per million (ppm)
View SolutionMole Fraction |Exercise Questions|Parts Per Million (ppm) / Parts Per Billion (ppb)|Exercise Questions|Summary
View SolutionMole Fraction|Exercise Questions|Parts Per Million (PPM) / Parts Per Billion (PPB)|Exercise Questions|Summary
View SolutionUnits of parts per million (ppm) or per billion (ppb) are often used to describe the concentrations of solutes in very dilute solutions. The units are defined as the number of grams of solution per million or per billion gram of solvent. Bay of Bengal has 2.1 ppm of lithium ions. What is the molality of Li^(+) in this water ? (Li = 7)
View SolutionAir contains about 0.03% of carbon dioxide. The concentration in parts per million is
View Solution

