The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In gaseous state, the enrgy required for hololytic cleavage of a bond is called Bond Dissociation Energy(BDE) or Bond Strength. BDE is affected by s-character of the bond and the stability of the radicals formed. Shorter bonds typically stronger bonds. BDEs for some bonds are given below: 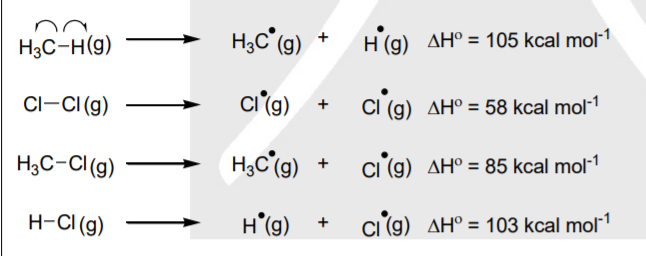
Correct match of the C-H bonds(shown in bold) in column J with their BDE in column K is 
P-iii,Q-iv,R-ii,S-i
P-i,Q-ii,R-iii,S-iv
P-iii,Q-ii.R-i,S-iv
P-ii,Q-i,R-iv,S-iii
Step by step video solution for The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In gaseous state, the enrgy required for hololytic cleavage of a bond is called Bond Dissociation Energy(BDE) or Bond Strength. BDE is affected by s-character of the bond and the stability of the radicals formed. Shorter bonds typically stronger bonds. BDEs for some bonds are given below: Correct match of the C-H bonds(shown in bold) in column J with their BDE in column K is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
A solution of 0 .1 weak base (B )is titrated with 0.1 M of a strong acid ( HA) . The variation of pH of the solution will be the volume of HA added is shown in the figure below. What is the pKb of the base ? The neutralization reaction is given by B+HA→HA→BH++A−
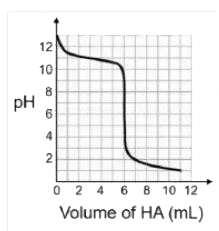
View SolutionMatch the rate expressions in LIST-I for the decomposition of X with the corresponding profiles provided in LIST-II. Xs and k are constants having appropriate units.

View SolutionAssertion (A): Bond energy of CI-CI bond is more than F-F bond.
Reason (R ): Shorter the bond length, stronger the bond, more is the bond energy.
View SolutionWhich of the following bonds require the largest amount of bond energy to dissociate the atoms concerned ?
View SolutionDifferent types of bond are formed in the chemical compound. These bond have different strength and bond energies associated with them. These bond are formed with atoms in different enviroments. Which of the following bond has highest bond energy ?
View SolutionWhy is more energy required to break a sigma bond than a pi bond?
View SolutionTotal energy required to break all the bonds in the gaseous state molecule of AB2 is xkJmol−1. Then the bond energy of the bond A-B in the molecule will be ________.
View Solutionआबन्ध वियोजन ऊर्जा या बंध एन्थैल्पी किसे कहते है ?
View SolutionBond breaking releaser energy whereas bond formation absorbs energy
View Solutionदिये गये परमाणुओं में किस बन्ध को तोड़ने ऊर्जा की मात्रा के लिये अधिकतम ऊर्जा की मात्रा आवश्यक होगी
View SolutionBond energy : Energy required to break a bond (equal to the energy released when a bond is formed) is named bond energy. Its unit is Kcal/mole. Stability of bond energy 00 % s character oo(1)/(% "P character")oo "bond order" oo(1)/("bond length") Higher is the number of bonds at a place, higher is the bond energy. Write down bond energy order of C- H bond in (A) -C- H, (B) = C-H, (C) = C - H
View SolutionWhich of the following bonds require the largest amount of energy to dissociate the bond concerned ?
View SolutionTotal energy required to break all the bonds in the gaseous state molecule of AB2 is xkJmol−1. Then the bond energy of the bond A-B in the molecule will be ________.
View SolutionWhich of the following bonds require the largest amount of bond energy to dissociate the atoms concerned ?
View SolutionBond Energy and Bond dissociation energy || Resonance energy
View SolutionAssertion : The bond energy and bond formation energy of a particular bond are equal in magnitude but opposite in sign.
Reason : The process of bond formation requires absorption of energy but the process of breaking of bond involves liberation of energy.
View Solution

